909637
NanoFabTx™ PLGA-nano
for synthesis of 100 and 200 nm particles
Synonym(s):
NanoFabTx™, NanoFabTx™ reagent kit, PLGA nanoformulation kit
About This Item
Recommended Products
description
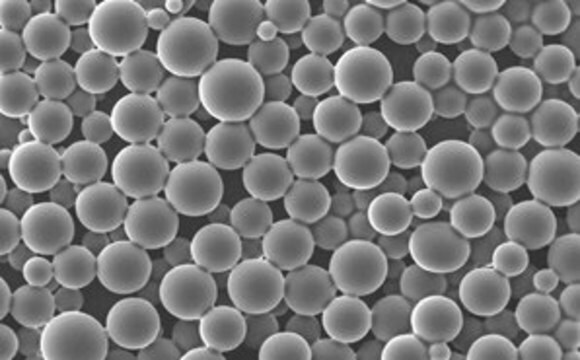
PLGA nanoparticle screening kit for synthesis of 100 and 200 nm particles
Quality Level
application(s)
advanced drug delivery
storage temp.
2-8°C
Related Categories
General description
Application
Features and Benefits
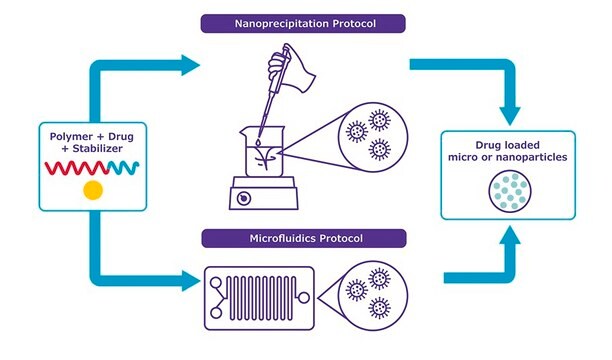
- Step-by-step protocols developed and tested by our formulation scientists
- Flexible synthesis tool to create uniform and reproducible nanoparticles
- Choose from standard glassware-based nanoprecipitation or microfluidic-based protocols
- Optimized to make nanoparticles 75-200 nm nanoprecipitation or microfluidics with low polydispersity
- Based on non-toxic, biodegradable polymers
Legal Information
related product
Storage Class Code
11 - Combustible Solids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Please refer to KIT Component information
PRTR
Please refer to KIT Component information
FSL
Please refer to KIT Component information
ISHL Indicated Name
Please refer to KIT Component information
ISHL Notified Names
Please refer to KIT Component information
Cartagena Act
Please refer to KIT Component information
JAN Code
キットコンポーネントの情報を参照してください
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service