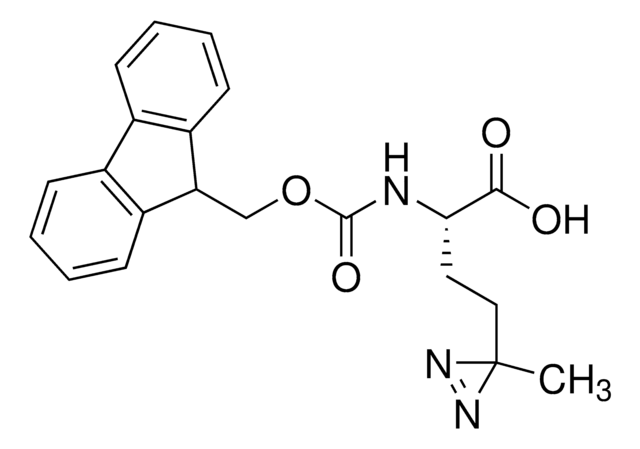
907340
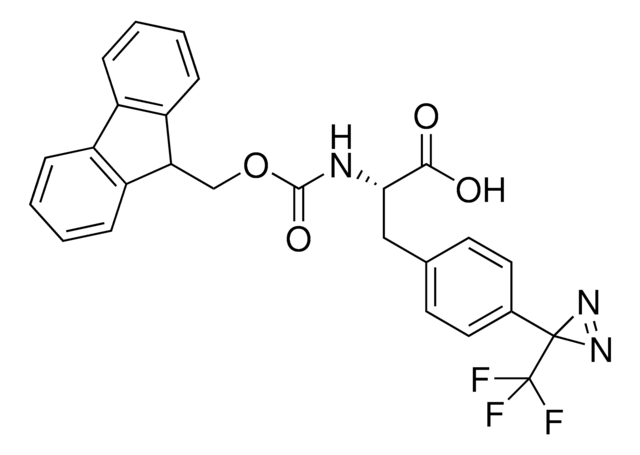
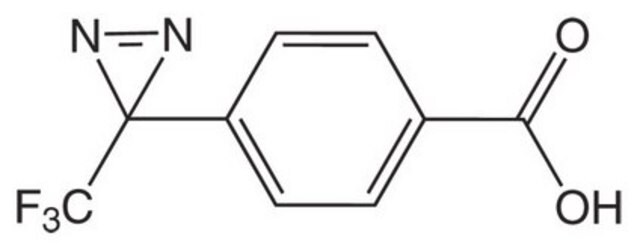
H-L-Photo-Phe-OH
≥98%
Synonym(s):
(S)-2-Amino-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, 4-(Trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, H-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Recommended Products
Assay
≥98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
availability
available only in USA
application(s)
peptide synthesis
storage temp.
−20°C
InChI
1S/C11H10F3N3O2/c12-11(13,14)10(16-17-10)7-3-1-6(2-4-7)5-8(15)9(18)19/h1-4,8H,5,15H2,(H,18,19)
InChI key
HRGXDARRSCSGOG-UHFFFAOYSA-N
Related Categories
Application
photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907294.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
A genetically encoded diazirine photo-crosslinker in Escherichia coli
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
907340-BULK:
907340-50MG:
907340-VAR:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service