803650
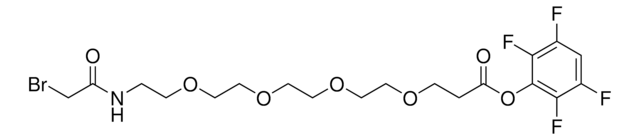
SBAP (succinimidyl 3-(bromoacetamido)propionate)
About This Item
Recommended Products
Assay
≥90%
Quality Level
form
powder
mol wt
307.1
reaction suitability
reagent type: cross-linking reagent
storage condition
desiccated
solubility
DMSO or DMF: soluble
functional group
NHS ester
bromo
shipped in
ambient
storage temp.
2-8°C
SMILES string
O=C(CCC1=O)N1OC(CCNC(CBr)=O)=O
InChI
1S/C9H11BrN2O5/c10-5-6(13)11-4-3-9(16)17-12-7(14)1-2-8(12)15/h1-5H2,(H,11,13)
InChI key
WGMMKWFUXPMTRW-UHFFFAOYSA-N
General description
Features and Benefits
- Reactive groups: NHS ester and bromoacetyl
- Reactive toward: amino and sulfhydryl groups
- NHS ester reacts with primary amines at pH 7-9 to form a stable amide bond
- Bromacetyl group reacts with sulfhydryl groups at pH > 7.5 to form stable thioether bonds
- Non-cleavable
- Water-insoluble (dissolve first in DMF or DMSO)
- Spacer maintains peptide-like character in the crosslinked species
- Resulting crosslink is susceptible to acid hydrolysis
- Useful for preparing cyclic peptides and peptide conjugates
Caution
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
803650-50MG:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service