790389
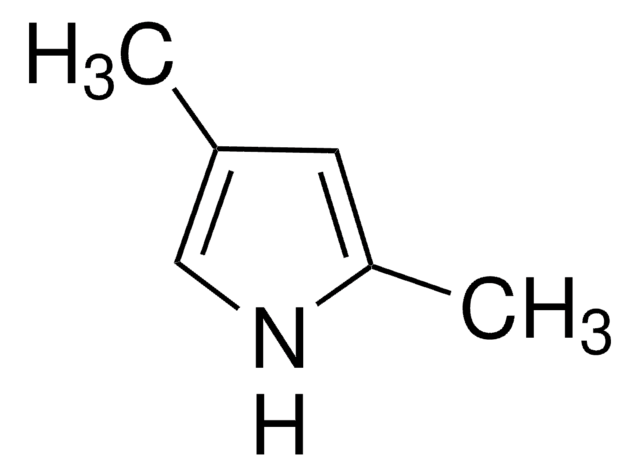
Difluoro{2-[1-(3,5-dimethyl-2H-pyrrol-2-ylidene-N)ethyl]-3,5-dimethyl-1H-pyrrolato-N}boron
99% (HPLC)
Synonym(s):
1,3,5,7,8-Pentamethyl-4,4-difluorro-4-bora-3a,4a-diaza-s-indacene, 2-[1-(3,5-Dimethyl-2H-pyrrol-2-ylidene)ethyl]-3,,5-dimethyl-1H-pyrrole, boron complex, 4,4-Difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene, BODIPY 493/503, PM 546, PMPBF2, Pyrromethene 546, [[2-[1-(3,5-Dimethyl-2H-pyrrol-2-ylidene)ethyl]-3,5-dimethyl-1H-pyrrolato-N1,N2]difluoro]boron
About This Item
Recommended Products
Quality Level
Assay
99% (HPLC)
form
powder
mp
262-266 °C
fluorescence
λex 493 nm; λem 504 nm in methanol
SMILES string
CC(C1=C(C)C=C(C)N1B(F)2F)=C3[N]2=C(C)C=C3C
InChI
1S/C14H17BF2N2/c1-8-6-10(3)18-13(8)12(5)14-9(2)7-11(4)19(14)15(18,16)17/h6-7H,1-5H3
InChI key
DRJHPEGNOPSARR-UHFFFAOYSA-N
Related Categories
General description
Application
Also used for solid-state dye laser devices and organic solar cells.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
790389-VAR:
790389-BULK:
790389-500MG:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| R571067-1EA | |
| 790389-500MG | 4061832948003 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service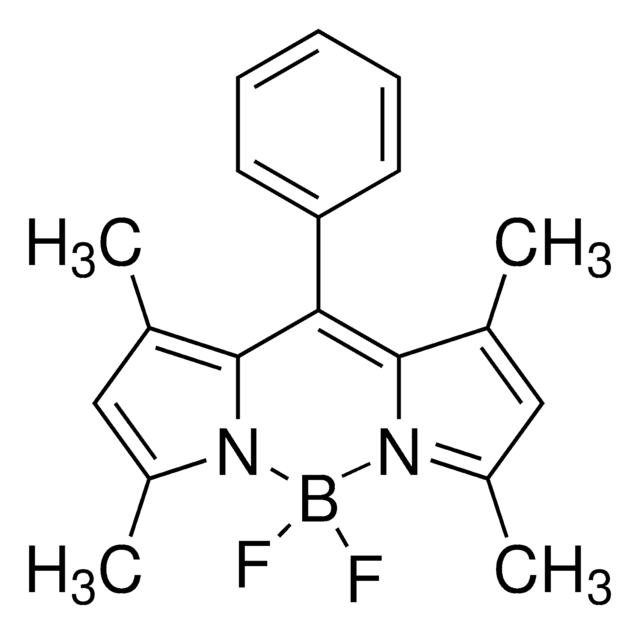
![Difluoro{2-[(3,5-dimethyl-2H-pyrrol-2-ylidene-N)methyl]-3,5-dimethyl-1H-pyrrolato-N}boron 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/518/861/c19c64be-654e-472e-a069-30ffccb1a8cd/640/c19c64be-654e-472e-a069-30ffccb1a8cd.png)

![Difluoro(4-(1,1-dimethylethyl)-2-{1-[4-(1,1-dimethylethyl)-3,5-dimethyl-2H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrolato-N)boron 98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/879/8046aafd-78ca-4fd8-92dc-801de0b6cc53/640/8046aafd-78ca-4fd8-92dc-801de0b6cc53.png)