733520
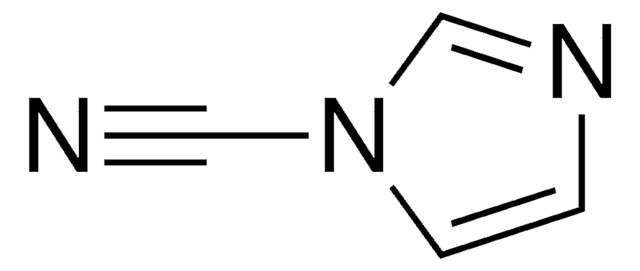
1-Cyanobenzimidazole
96%
Synonym(s):
N-Cyanobenzimidazole
About This Item
Recommended Products
Quality Level
Assay
96%
form
solid
reaction suitability
reaction type: C-C Bond Formation
mp
98-106 °C
SMILES string
N#Cn1cnc2ccccc12
InChI
1S/C8H5N3/c9-5-11-6-10-7-3-1-2-4-8(7)11/h1-4,6H
InChI key
SGCJHVTWXFWDOD-UHFFFAOYSA-N
Application
- To prepare alkyl benzimidazole-1-carboximidates and 1H-benzimidazole-1-carbohydrazonamide by reacting with aliphatic alcohols and excess of hydrazine, respectively.
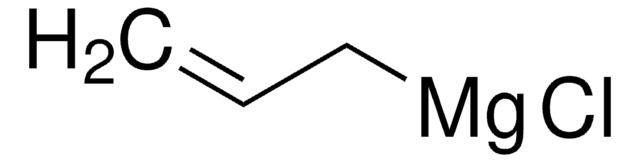
- As an electrophilic cyanating reagent in cyanation reactions including aryl and heteroaryl Grignard reagents.
- Electrophilic aromatic substitution reactions
- Electrophilic cyanation of aryl and heteroaryl Grignard reagents
- Hydrolysis in alkalyne solutions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
733520-VAR:
733520-BULK:
733520-1G:
733520-5G:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service