683876
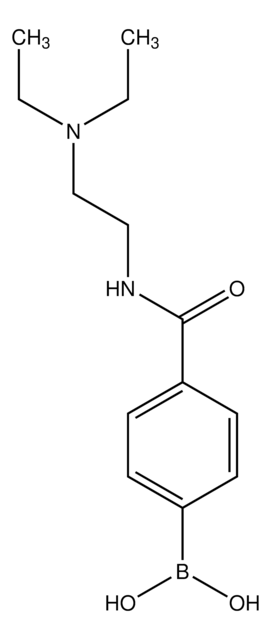
4-Aminocarbonylphenylboronic acid
≥95%
Synonym(s):
4-Carbamoylbenzeneboronic acid, 4-Carbamoylphenylboronic acid
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder or crystals
mp
229-234 °C
functional group
amide
storage temp.
2-8°C
SMILES string
NC(=O)c1ccc(cc1)B(O)O
InChI
1S/C7H8BNO3/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,11-12H,(H2,9,10)
InChI key
GNRHNKBJNUVWFZ-UHFFFAOYSA-N
Related Categories
Application
- Suzuki-Miyaura, Sonogashira and Buchwald-Hartwig cross-coupling reactions for the synthesis of substituted pyrene derivatives
- Suzuki-Miyaura reactions for the synthesis of aryl-substituted oxabenzindoles and methanobenzindoles or 2-aminoimidazole triazoles
- Three-component coupling with triflates and alkenes
Reactant involved in synthesis of biologically active molecules including:
- Hybrid peptidomimetic molecules as STAT3 protein inhibitors
- Vasopressin V1B receptor antagonists for use as antidepressants oand anxiolytics
- (Thienopyridine)caboxamides as CHK1 inhibitors
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
683876-5G:
683876-BULK:
683876-VAR:
683876-1G:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[3-(2-carboxyethyl)phenyl]boronic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/265/067/68263dcf-5afc-49a6-982b-0394e48bf9c2/640/68263dcf-5afc-49a6-982b-0394e48bf9c2.png)