556432
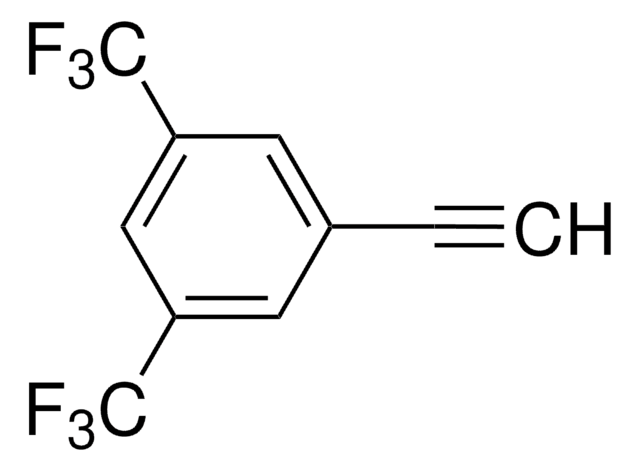
4-Ethynyl-α,α,α-trifluorotoluene
97%
Synonym(s):
1-Ethynyl-4-(trifluoromethyl)benzen, 1-Ethynyl-4-(trifluoromethyl)benzene, 1-Trifluoromethyl-4-ethynylbenzene, 4-(Trifluoromethyl)phenylacetylene, 4-Ethynyl(trifluoromethyl)benzene, 4-Ethynyl-trifluorotoluene, 4-Trifluorophenylacetylene, [4-(Trifluoromethyl)phenyl]ethyne, p-(Trifluoromethyl)phenylacetylene
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.4650 (lit.)
bp
78-80 °C/2 mmHg (lit.)
density
1.043 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
FC(F)(F)c1ccc(cc1)C#C
InChI
1S/C9H5F3/c1-2-7-3-5-8(6-4-7)9(10,11)12/h1,3-6H
InChI key
XTKBMZQCDBHHKY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
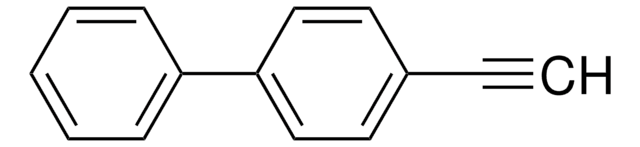
- 6,13-bis(4-trifluoromethylphenylethynyl)pentacene
- 1,2-dialkynylimidazoles
- trans-[Co(cyclam)(p-CCC6H4CF3)2]OTf complex (where cyclam - 1,4,8,11-tetraazacyclotetradecane; 4-ethynyl-α,α,α-trifluorotoluene - p-CCC6H4CF3; OTf- trifluoromethane sulfonate)
- 3-spiroazetidinimine-2-oxindoles
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
69.1 °F - closed cup
Flash Point(C)
20.6 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 1 petroleums
Hazardous rank II
Water insoluble liquid
JAN Code
556432-5G:
556432-BULK:
556432-VAR:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The terminal alkyne functionality has a wide range of applications including most recently the synthesis of spiropyran substituted 2,3-dicyanopyrazines and (±)-asteriscanolide, as well as conversion to enamines using resin-bound 2° amines.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| R700266-1EA | |
| 556432-5G | 4061832582757 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![1-[(Trimethylsilyl)ethynyl]-4-(trifluoromethyl)benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/738/524/1dbb534f-6783-4963-ac51-9bf29c099aaa/640/1dbb534f-6783-4963-ac51-9bf29c099aaa.png)