All Photos(2)
About This Item
Empirical Formula (Hill Notation):
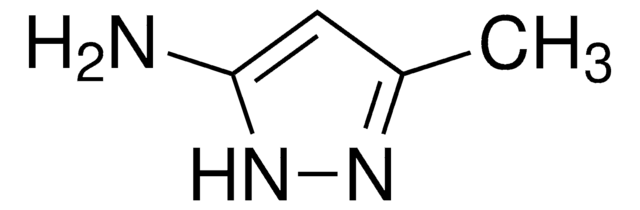
C10H11N3
CAS Number:
Molecular Weight:
173.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
114-117 °C (lit.)
SMILES string
Cc1cc(N)n(n1)-c2ccccc2
InChI
1S/C10H11N3/c1-8-7-10(11)13(12-8)9-5-3-2-4-6-9/h2-7H,11H2,1H3
InChI key
FMKMKBLHMONXJM-UHFFFAOYSA-N
Related Categories
General description
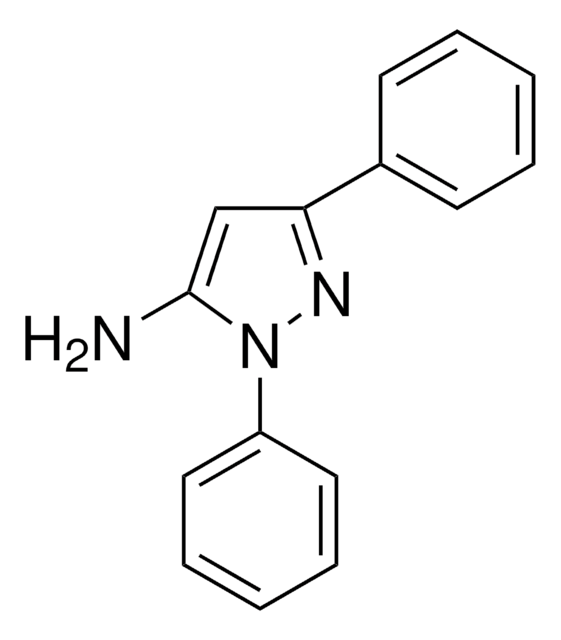
5-Amino-3-methyl-1-phenylpyrazole is an aminopyrazole derivative. It reacts with 6-methyl-4-oxo-4H-[1]-benzopyran-3-carboxaldehyde to yield 5-(2-hydroxy-5-methylbenzoyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine and 2-methoxy-6-methyl-3-(3-methyl-1-phenylpyrazol-5-ylaminomethylene)chroman-4-one.
Application
5-Amino-3-methyl-1-phenylpyrazole may be used to synthesize:
- substituted pyrazoles
- pyrazolopyridine derivatives
- pyrazolo[3,4,-b]pyridines
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The synthesis of fused and pendant pyrazole heterocyclic compounds from 5-amino-3-methyl-1-phenylpyrazole and their evaluation as fluorescent brightening agents.
Tagdiwala PV and Rangnekar DW.
Journal of Chemical Technology and Biotechnology, 38(2), 77-84 (1987)
Synthesis of Newly Substituted Pyrazoles and Substituted Pyrazolo [3, 4-b] Pyridines Based on 5-Amino-3-Methyl-1-Phenylpyrazole.
El-Emary TI.
J. Chin. Chem. Soc., 54(2), 507-518 (2007)
An unexpected chemical behavior of 5-N-(benzotriazol-1-ylmethyl) amino-3-tert-butyl-1-phenylpyrazole.
Abonia R, et al.
Tetrahedron Letters, 43(22), 5617-5620 (2002)
Transformation of 4-oxo-4H-[1]-benzopyran-3-carboxaldehydes into pyrazolo [3, 4-B] pyridines.
Stankovicova H, et al.
Journal of Heterocyclic Chemistry, 43(4), 843-848 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service