About This Item
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.483 (lit.)
bp
277 °C (lit.)
density
1.082 g/mL at 25 °C (lit.)
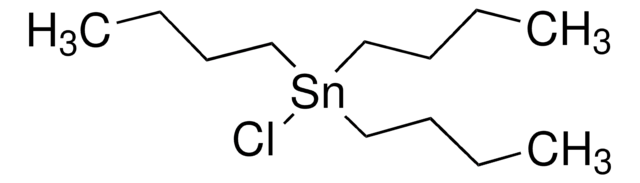
SMILES string
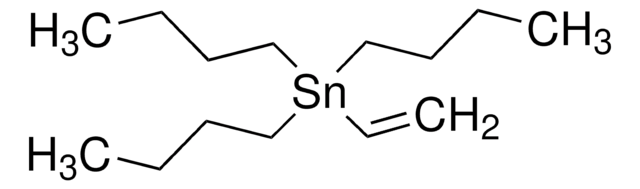
CCCC[Sn](CCCC)(CCCC)C#CC
InChI
1S/3C4H9.C3H3.Sn/c3*1-3-4-2;1-3-2;/h3*1,3-4H2,2H3;1H3;
InChI key
KCQJLTOSSVXOCC-UHFFFAOYSA-N
Application
- As a starting material in the synthesis of phenyl-substituted pyrimidine-dione by reacting with ethyl isocyanate via cycloaddition and Stille coupling reactions.
- In the synthesis of 18F labeled triazolo quinoline derivative, a positron emission tomography (PET) ligand used in the in vivo imaging of metabotropic glutamate receptor type 1.
- In one of the key synthetic steps for the synthesis of 4-(1-aryltriazol-4-yl)-tetrahydropyridines as potent mGluR1 antagonists.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
499862-5G:4548173308760
499862-VAR:
499862-1G:4548173308753
499862-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service