425664
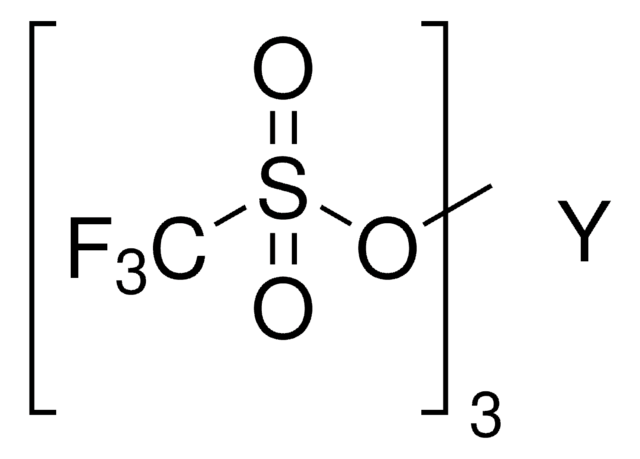
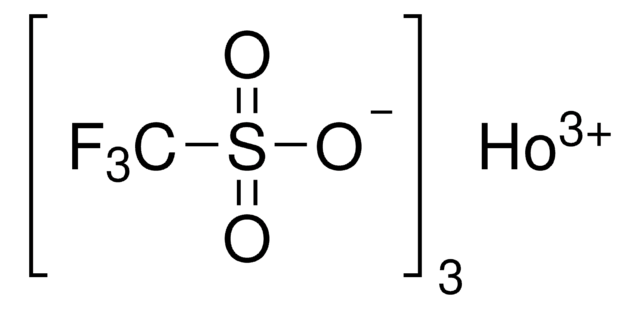
Dysprosium(III) trifluoromethanesulfonate
98%
Synonym(s):
Dysprosium(III) triflate, Tris(triflato)dysprosium
About This Item
Recommended Products
Assay
98%
reaction suitability
core: dysprosium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
SMILES string
[Dy+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Dy/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
XSVCYDUEICANRJ-UHFFFAOYSA-K
General description
Application
- Aza-Piancatelli rearrangement
- Friedel-Crafts alkylation
- Ring-opening polymerization reactions
- Microwave-assisted Kabachnik-Fields condensation
- Cycloaddition reactions (Lewis-acid catalyst)
- Fries rearrangement
- Enantioselective glyoxalate-ene reactions
- Aldol reaction of silyl enol ethers with aldehydes.
- As an effective catalyst for electrophilic substitution reactions of indoles with imines.
- As catalyst for the synthesis of 4-aminocyclopentenones and functionalized azaspirocycles, via intramolecular aza-Piancatelli rearrangement.
- As new curing initiator to study the curing of diglycidyl ether of bisphenol-A (DGEBA).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
425664-5G:
425664-25G:
425664-BULK:
425664-VAR:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service