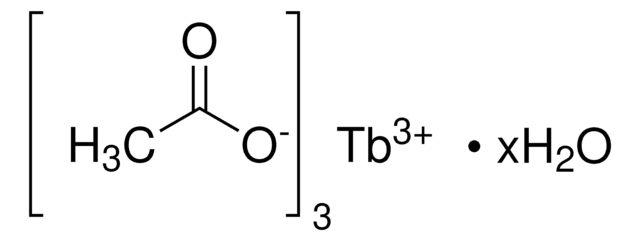
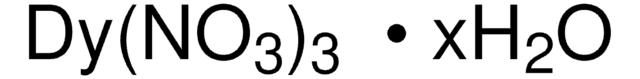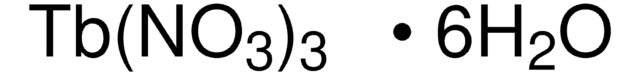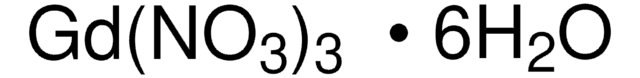325945
Terbium(III) nitrate pentahydrate
99.9% trace metals basis
Synonym(s):
Terbium nitrate pentahydrate, Terbium(3+) nitrate pentahydrate
About This Item
Recommended Products
grade
for analytical purposes
Assay
99.9% trace metals basis
form
solid
reaction suitability
reagent type: catalyst
core: terbium
impurities
≤1500.0 ppm Trace Rare Earth Analysis
SMILES string
O.O.O.O.O.[Tb+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/3NO3.5H2O.Tb/c3*2-1(3)4;;;;;;/h;;;5*1H2;/q3*-1;;;;;;+3
InChI key
YWROXJNVUWBEPC-UHFFFAOYSA-N
General description
Application
- A precursor to prepare Ce-doped terbium aluminum garnets by the photo-induced method.
- A dopant to synthesize emission-tunable luminescent hydroxyapatite probe for bioimaging.
- A starting material to prepare metal-organic frameworks for green laser applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 1: Oxidizing solids
Nitrates
Hazardous rank I
1st oxidizing solid
JAN Code
325945-BULK:
325945-VAR:
325945-5G:
325945-25G:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service