About This Item
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.498 (lit.)
bp
152-153 °C (lit.)
density
0.984 g/mL at 25 °C (lit.)
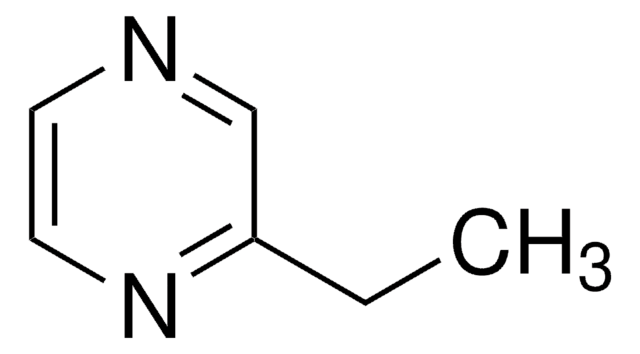
SMILES string
CCc1cnccn1
InChI
1S/C6H8N2/c1-2-6-5-7-3-4-8-6/h3-5H,2H2,1H3
InChI key
KVFIJIWMDBAGDP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
109.0 °F
Flash Point(C)
42.78 °C
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Flammable liquids
Type 2 petroleums
Hazardous rank III
Water insoluble liquid
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Separation of 2-Ethyl-3-methylpyrazine; 1-Methylpyrrole; 2,3-Dimethylpyrazine; 2,5-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Diethylpyrazine; 2-Methylpyrazine; Carbon disulfide; Dimethyl disulfide; 2,6-Dimethylpyrazine
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service