185922
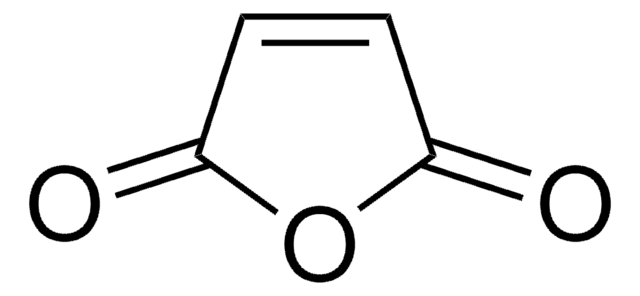
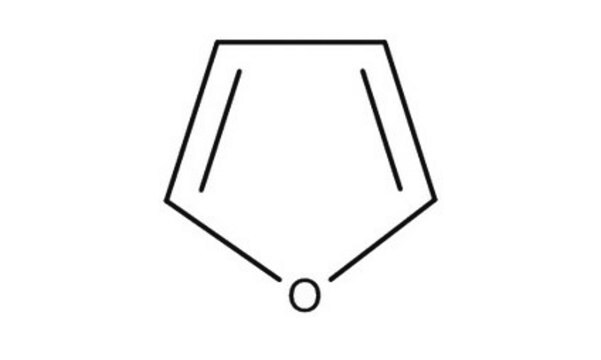
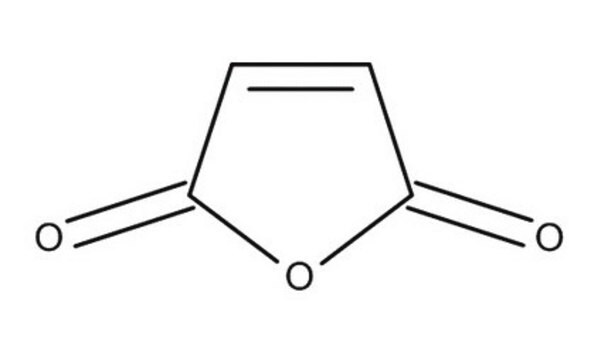
Furan
≥99%
Synonym(s):
1,4-Epoxybuta-1,3-diene, Divinylene oxide, Oxacyclopentadiene, Oxole, Tetrole
About This Item
Recommended Products
vapor density
2.35 (vs air)
Quality Level
vapor pressure
1672 mmHg ( 55 °C)
31.66 psi ( 55 °C)
493 mmHg ( 20 °C)
9.22 psi ( 20 °C)
Assay
≥99%
form
liquid
contains
0.025 wt. % BHT as inhibitor
expl. lim.
14.3 %
refractive index
n20/D 1.421 (lit.)
bp
32 °C/758 mmHg (lit.)
solubility
alcohols: freely soluble
diethyl ether: freely soluble
water: insoluble
density
0.936 g/mL at 25 °C (lit.)
shipped in
wet ice
storage temp.
2-8°C
SMILES string
c1ccoc1
InChI
1S/C4H4O/c1-2-4-5-3-1/h1-4H
InChI key
YLQBMQCUIZJEEH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Preparation of the starting material required for the synthesis of calix[6]pyrrole.
- To investigate the kinetics and mechanism of reactions of chlorine atoms with volatile organic compounds.
- Catalytic transformation of furan to aromatics and olefins.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 1B - Flam. Liq. 1 - Muta. 2 - Skin Irrit. 2 - STOT RE 2
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-32.8 °F - closed cup
Flash Point(C)
-36 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class II Designated Chemical Substances
FSL
Group 4: Flammable liquids
Special flammables
Hazardous rank I
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
185922-BULK:
185922-100ML:
185922-10L:
185922-5L:
185922-5ML:
185922-VAR:
185922-500ML:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service