184802
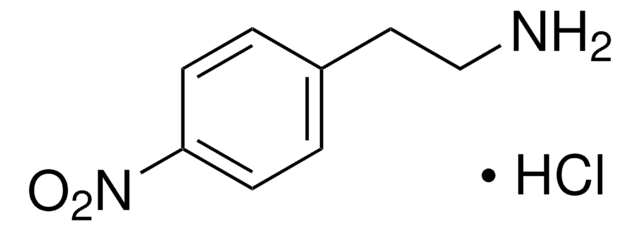
4-Nitrophenethylamine hydrochloride
95%
Synonym(s):
2-(4-Nitrophenyl)ethylamine hydrochloride
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
mp
200 °C (dec.) (lit.)
functional group
amine
nitro
SMILES string
Cl.NCCc1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H10N2O2.ClH/c9-6-5-7-1-3-8(4-2-7)10(11)12;/h1-4H,5-6,9H2;1H
InChI key
JVMHULJEYUQYSH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- ortho-metalated primary phenethylamines complexes containing six-membered palladacycles
- N-(4-nitrophenethyl)formamide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
184802-BULK:
184802-VAR:
184802-5G:
184802-1G:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 184802-1G | |
| 184802-5G | 4061838756770 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service