175501
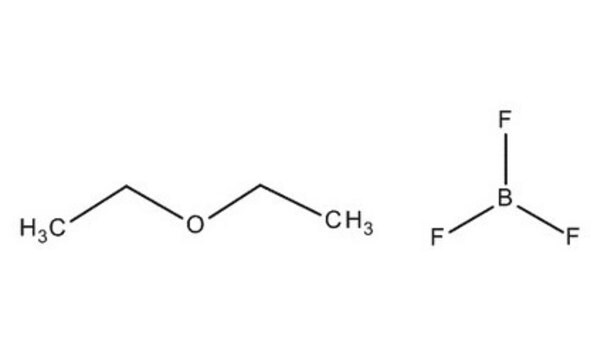
Boron trifluoride diethyl etherate
for synthesis
Synonym(s):
Boron trifluoride ethyl etherate
About This Item
Recommended Products
grade
for synthesis
synthesis grade
Quality Level
vapor density
4.9 (vs air)
vapor pressure
4.2 mmHg ( 20 °C)
form
liquid
expl. lim.
36 %
reaction suitability
core: boron
reagent type: Lewis acid
reagent type: catalyst
refractive index
n20/D 1.344 (lit.)
bp
126-129 °C (lit.)
mp
−58 °C (lit.)
density
1.15 g/mL (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
CC[O+](CC)[B-](F)(F)F
InChI
1S/C4H10BF3O/c1-3-9(4-2)5(6,7)8/h3-4H2,1-2H3
InChI key
MZTVMRUDEFSYGQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Packaging
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT RE 1 Inhalation
Target Organs
Kidney
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
137.3 °F - closed cup
Flash Point(C)
58.5 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
FSL
Group 4: Flammable liquids
Type 2 petroleums
Hazardous rank III
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
175501-1L:
175501-BULK:
175501-4X25ML:
175501-PZ:
175501-100ML:
175501-500ML:
175501-VAR:
175501-4L:
175501-5ML:
175501-2L:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 175501-2L | |
| 175501-4L | 4061838252159 |
| 175501-500ML | |
| 175501-100ML | 4061838752314 |
| 175501-1L | 4061838252142 |
| 175501-4X25ML | 4061838252166 |
| 175501-5ML | 4061838752321 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service