162957
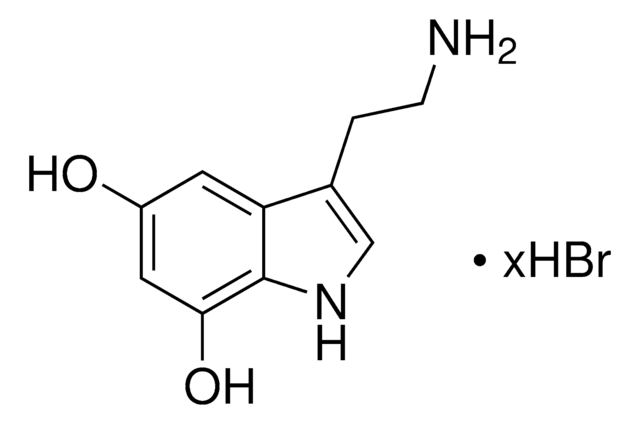
6-Hydroxydopamine hydrobromide
95% (HPLC), powder, neurotoxin
Synonym(s):
2,4,5-Trihydroxyphenethylamine hydrobromide, 2,5-Dihydroxytyramine hydrobromide, 2-(2,4,5-Trihydroxyphenyl)ethylamine hydrobromide, 6-OHDA
About This Item
Recommended Products
Product Name
6-Hydroxydopamine hydrobromide, 95%
Assay
95%
form
powder
mp
216-220 °C (lit.)
storage temp.
−20°C
SMILES string
Br.NCCc1cc(O)c(O)cc1O
InChI
1S/C8H11NO3.BrH/c9-2-1-5-3-7(11)8(12)4-6(5)10;/h3-4,10-12H,1-2,9H2;1H
InChI key
MLACDGUOKDOLGC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to induce Parkinson′s disease (PD) in mouse models to study the effects of tubastatin A (TBA) on nucleotide-binding oligomerization domain and leucine-rich repeat pyrin 3 domain (NLRP3) activation and cell injury in SH-SY5Y cells
- to induce pharmacological ablation of the sympathetic nerves to study the effect of hepatic sympathetic nerve activity (SNA) on hepatic steatosis during diet-induced obesity in mice
- to induce oxidative stress in mesencephalic cells to study its effect on p75NTR signaling in neuronal cells of the ventral mesencephalon
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
162957-250MG:
162957-1G:
162957-50MG:
162957-BULK:
162957-VAR:
162957-5G:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service