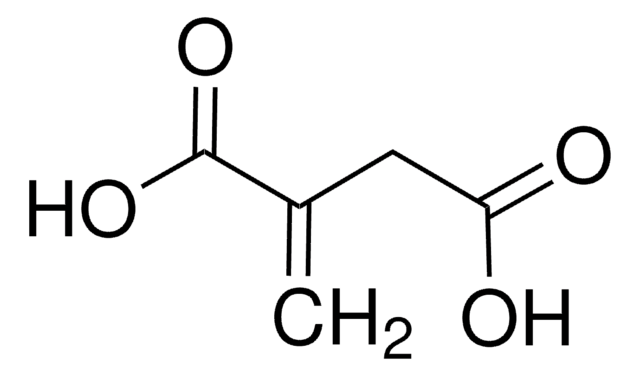
151076
9-Borabicyclo[3.3.1]nonane solution
0.5 M in THF
Synonym(s):
9-BBN
About This Item
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: reductant
concentration
0.5 M in THF
density
0.894 g/mL at 25 °C
SMILES string
B1C2CCCC1CCC2
InChI
1S/C8H15B/c1-3-7-5-2-6-8(4-1)9-7/h7-9H,1-6H2/t7-,8+
InChI key
FEJUGLKDZJDVFY-OCAPTIKFSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Reactant for:
- Linear SPPS synthesis of ubiquitin derivatives
- Copper-catalyzed cross-coupling reactions of organoboron compounds with primary alkyl halides and pseudohalides
- Intramolecular insertion of alkenes into palladium-nitrogen bonds
- Preparation of (phosphonoacetyl)ornithine to study effect on arginine biosynthetic genes in yeast
- Hetero-Diels-Alder reaction for synthesis of spirocyclic alkaloids
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3 - Water-react 1
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
1.0 °F - closed cup
Flash Point(C)
-17.2 °C - closed cup
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
FSL
Group 4: Flammable liquids
Type 1 petroleums
Hazardous rank II
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
151076-18L:4548173106151
151076-100ML:4548173106144
151076-VAR:
151076-PZ:
151076-20L:
151076-4X25ML:4548173310459
151076-18L-C:
151076-18L-KL:
151076-BULK:
151076-800ML:4548173106168
151076-8L:4548173106175
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Tandem hydroboration Suzuki Coupling both intermolecular and intramolecular gave diverse alkyl substituted products dppf
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service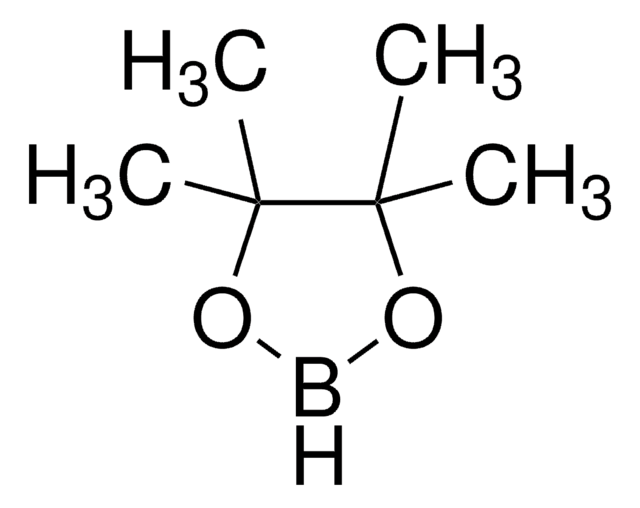

![9-Borabicyclo[3.3.1]nonane dimer](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)
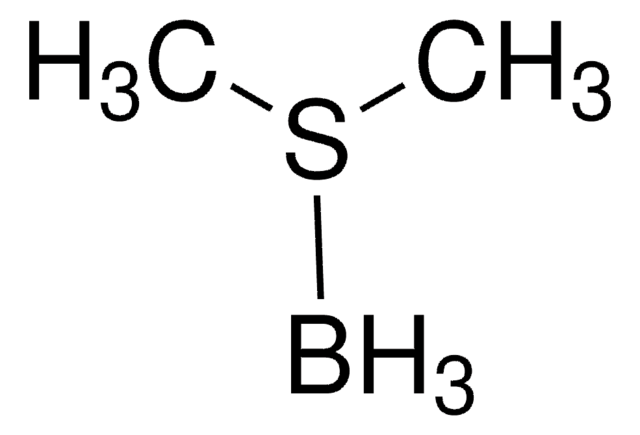

![9-Borabicyclo[3.3.1]nonane solution 0.4 M in hexanes](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)