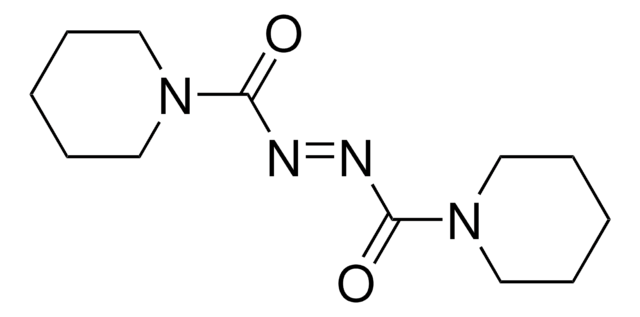
11627
Diethyl azodicarboxylate solution
purum, ~40% in toluene (H-NMR)
Synonym(s):
1,2-Ethoxycarbonyl diazene solution, DEAD, Diethoxycarbonyldiazene solution, Diethyl azodiformate solution, NSC 3474, NSC 679015, Unifoam AZ-AE 200
About This Item
Recommended Products
grade
purum
form
solid
concentration
~40% in toluene (H-NMR)
refractive index
n20/D 1.47
storage temp.
2-8°C
SMILES string
CCOC(=O)\N=N/C(=O)OCC
InChI
1S/C6H10N2O4/c1-3-11-5(9)7-8-6(10)12-4-2/h3-4H2,1-2H3/b8-7+
InChI key
FAMRKDQNMBBFBR-BQYQJAHWSA-N
Looking for similar products? Visit Product Comparison Guide
Application
DEAD can also be used as a reagent in the:
- Synthesis of esters, ethers, amines, and thioethers of alcohols.
- Oxidation of alcohols to carbonyl derivatives using ZnBr2 as a catalyst via dehydrogenation reaction.
- Conversion of alcohol to an azide key intermediate in the total synthesis of immunostimulant α-galactosylceramides.
- Synthesis of aza-β-lactams from aryl(alkyl)ketenes.
- Synthesis of 1H-1,2,4-triazole-1,4(5H)-dicarboxylate derivatives.
- Diels-Alder type reactions.
- Immunostimulants α-Galactosylceramides
- Cellotriose and cellotetraose analogues as transition state mimics for mechanistic studies of cellulases
- Bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase
- Derivatives of F200 and S383 with cannabinoid CB1 receptor binding activities
- Aza-β-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes
Reagent for:
- Annulation of N-protected imines
- α-thiocyanation of enolizable ketones with ammonium thiocyanate
- Diels-Alder reactions
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
105.8 °F - closed cup
Flash Point(C)
41 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
FSL
Group 4: Flammable liquids
Type 2 petroleums
Hazardous rank III
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
11627-50ML-F:
11627-VAR-F:
11627-250ML-F:
11627-500ML-F:
11627-BULK-F:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service