SMB00174
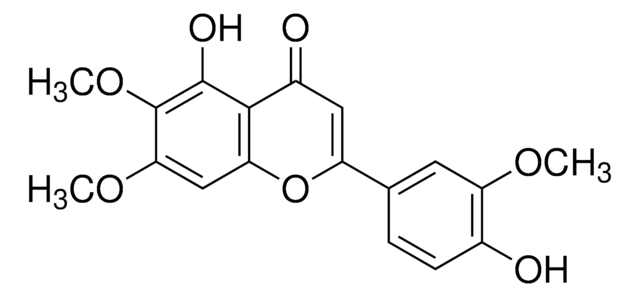
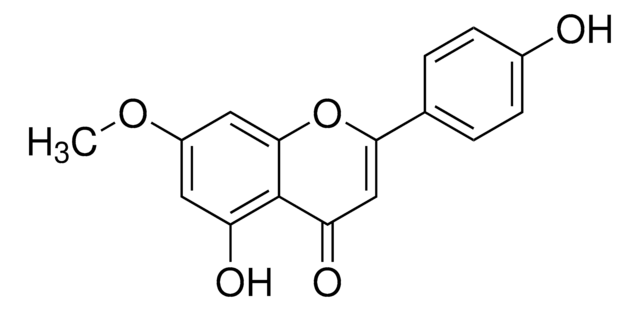
Cirsimaritin
≥90% (LC/MS-ELSD)
Sinonimo/i:
4′,5-Dihydroxy-6,7-dimethoxyflavone, Skrofulein
About This Item
Prodotti consigliati
Saggio
≥90% (LC/MS-ELSD)
Stato
solid
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Temperatura di conservazione
−20°C
Stringa SMILE
COc1cc2OC(=CC(=O)c2c(O)c1OC)c3ccc(O)cc3
InChI
1S/C17H14O6/c1-21-14-8-13-15(16(20)17(14)22-2)11(19)7-12(23-13)9-3-5-10(18)6-4-9/h3-8,18,20H,1-2H3
ZIIAJIWLQUVGHB-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Azioni biochim/fisiol
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| SMB00174-1MG | 4061837076220 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.