MSQC7
SILu™MAb K4 Stable-Isotope Labeled Universal Monoclonal Antibody
recombinant, expressed in CHO cells
Sinonimo/i:
SILu™MAB Stable-Isotope Labeled Universal Monoclonal Antibody Standard human, IgG4 kappa, Isotopically labeled mAb LC/MS Standard, Mass Spectrometry Monoclonal Antibody Standard
About This Item
Prodotti consigliati
Ricombinante
expressed in CHO cells
Livello qualitativo
Tipo di anticorpo
primary antibodies
Saggio
≥90% (SDS-PAGE)
Confezionamento
vial of 100 μg (± 10% Lot-specific vial content given on certificate of analysis)
Condizioni di spedizione
wet ice
Temperatura di conservazione
−20°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Caratteristiche e vantaggi
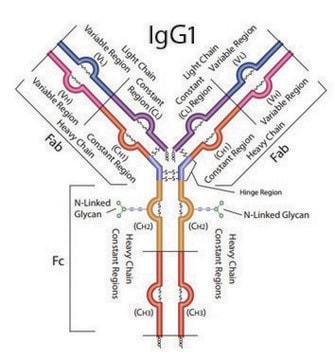
YGPPCPPCPAPEFLGGPSVFLFPPKPK Heavy Chain (IgG4 Stabilized)
VVSVLTVLHQDWLNGK Heavy Chain (IgG1, IgG3, IgG4)
GFYPSDIAVEWESNGQPENNYK Heavy Chain (IgG1, IgG4)
TTPPVLDSDGSFFLYSR Heavy Chain (IgG4)
SGTASVVCLLNNFYPR Light Chain (kappa)
VDNALQSGNSQESVTEQDSK Light Chain (kappa)
DSTYSLSSTLTLSK Light Chain (kappa)
Underlined is a Serine-Proline substitution in the hinge region that is present in many human IgG4-based therapeutic monoclonal antibodies
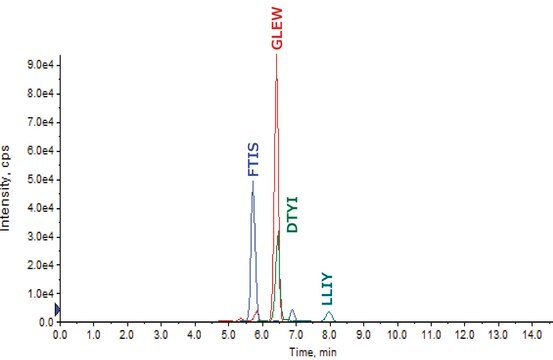
SILuMab has been validated as an internal standard for quantitation of relevant biotherapeutics in a complex biological matrix by MRM-based LC-MS/MS.
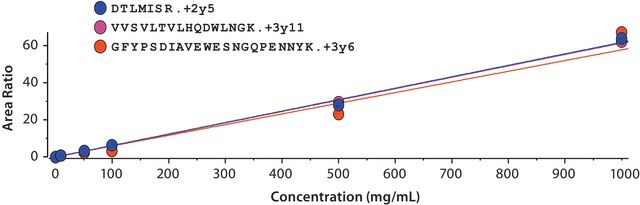
- SILuMab yielded reproducible, linear curves from 0.1 μg/mL to 1000 μg/mL without enrichment or depletion.
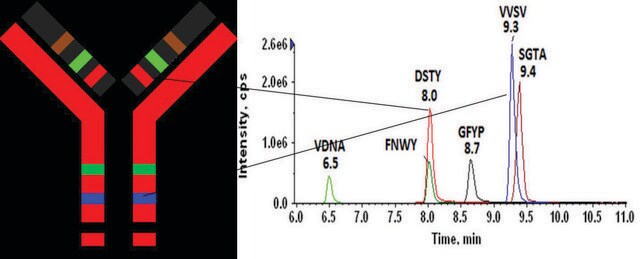
- Good agreement was observed between multiple peptides derived from the same target.
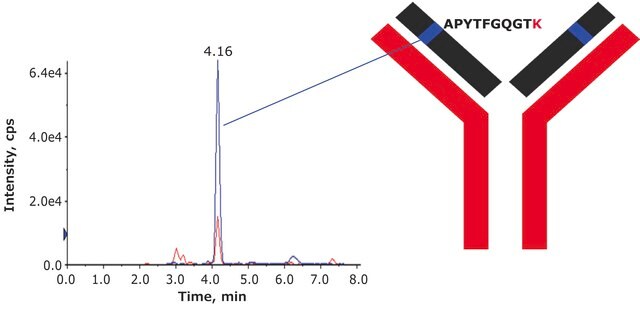
- Label incorporation was determined to be >98% by mass spectrometry.
- Sequence coverage was confirmed by peptide mapping.
Stato fisico
Nota sulla preparazione
Ricostituzione
Procedure
- Briefly centrifuge the vial at ~10,000 x g to collect the product at the bottom of the vial.
- Add 500 μL of purified water containing 0.1% formic acid to the vial.
- Mix the contents by gently inverting the vial a minimum of 5 times.
- Allow the vial to stand at room temperature for a minimum of 15 minutes and repeat mixing by inversion.
Risultati analitici
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKIGTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRWAPLGAFDIWGQGTMVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPPCPPCPAPEFLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLG
SILuMAb K4 Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIYDATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGVVFGGGTKLTVLTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
Target overlap areas are underlined
Package size based on protein content determined by A280 using an extinction coefficient (E0.1%) of 1.4
MRM settings provided (xls)
Note legali
Non trovi il prodotto giusto?
Prova il nostro Motore di ricerca dei prodotti.
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.