G2752
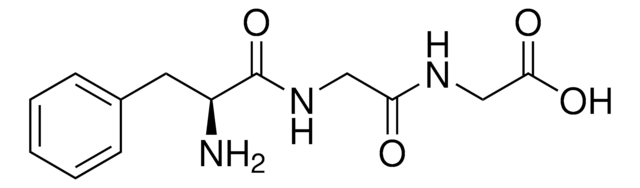
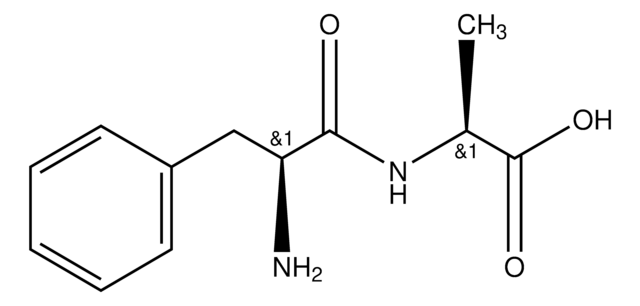
Gly-Phe
≥97.0% (TLC)
Sinonimo/i:
Glycyl-L-phenylalanine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NH2CH2CONHCH(COOH)CH2C6H5
Numero CAS:
Peso molecolare:
222.24
Beilstein:
2279318
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
Gly-Phe,
Saggio
≥97.0% (TLC)
Livello qualitativo
Stato
crystalline
Colore
white
Punto di fusione
264 °C
Temperatura di conservazione
−20°C
Stringa SMILE
NCC(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI
1S/C11H14N2O3/c12-7-10(14)13-9(11(15)16)6-8-4-2-1-3-5-8/h1-5,9H,6-7,12H2,(H,13,14)(H,15,16)/t9-/m0/s1
JBCLFWXMTIKCCB-VIFPVBQESA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
T Takarada et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 6(21), 3906-3913 (2000-12-29)
Oligopeptides are efficiently hydrolyzed by Ce(IV) to the corresponding amino acids under mild conditions. The pseudo first-order rate constants for the hydrolysis of H-Gly-Phe-OH and H-Gly-Gly-OH at pH 7.0 and 50 degrees C are 3.5 x 10(-1) and 2.8 x
Susan Weng Larsen et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 22(5), 399-408 (2004-07-22)
Oil-based depot formulations may constitute a future delivery method for small peptides. Thus, a requirement is attainment of sufficient oil solubility for such active compounds. A model dipeptide (Gly-Phe) has been converted into lipophilic prodrugs by esterification at the C-terminal
Kuniyo Inouye et al.
Bioscience, biotechnology, and biochemistry, 71(8), 2083-2086 (2007-08-11)
The aim of this study was to improve the performance of affinity gels containing glycyl-D-phenylalanine (Gly-D-Phe) as a ligand to thermolysin. Gly-D-Phe was immobilized to the resin through spacers of varying chain lengths. The resulting affinity gels had spacer chain
Roderick Y H Lim et al.
Proceedings of the National Academy of Sciences of the United States of America, 103(25), 9512-9517 (2006-06-14)
Natively unfolded phenylalanine-glycine (FG)-repeat domains are alleged to form the physical constituents of the selective barrier-gate in nuclear pore complexes during nucleocytoplasmic transport. Presently, the biophysical mechanism behind the selective gate remains speculative because of a lack of information regarding
S J Reshkin et al.
The American journal of physiology, 260(3 Pt 2), R563-R569 (1991-03-01)
The transport mechanisms for the dipeptide glycyl-L-phenylalanine (Gly-Phe) and L-phenylalanine (Phe) were characterized in fish intestinal brush-border membrane vesicles (BBMV). Gly-Phe was rapidly hydrolyzed only intravesicularly with almost total hydrolysis occurring even at 10 s. Dipeptide uptake was not stimulated
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.