C1613
Carbenicillin
Ready Made Solution, 100 mg/mL in ethanol/water, 0.2 μm filtered
Sinonimo/i:
α-Carboxybenzylpenicillin solution
About This Item
Prodotti consigliati
Livello qualitativo
Sterilità
0.2 μm filtered
Forma fisica
liquid
Concentrazione
100 mg/mL in ethanol/water
Solubilità
H2O: 100 mg/mL
ethanol: 100 mg/mL
Compatibilità
suitable for testing specifications (Bacteriostatic (cell type) E. Coli DH5a, result: pass)
Spettro attività antibiotica
Gram-negative bacteria
Gram-positive bacteria
Modalità d’azione
cell wall synthesis | interferes
Condizioni di spedizione
wet ice
Temperatura di conservazione
−20°C
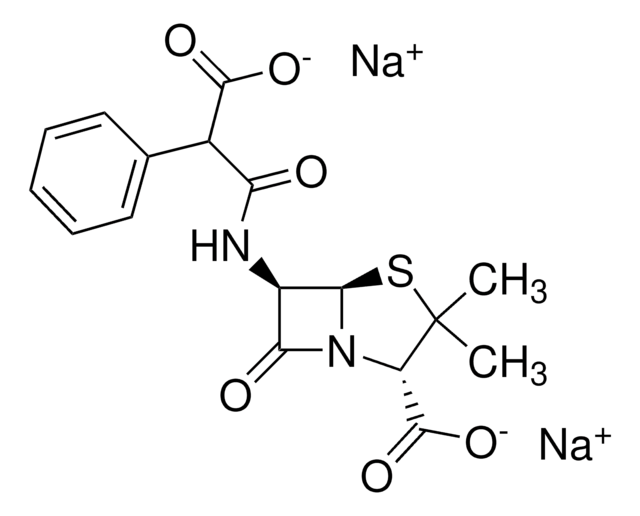
Stringa SMILE
CC1(C)S[C@@H]2[C@H](NC(=O)C(C(O)=O)c3ccccc3)C(=O)N2[C@H]1C(O)=O
InChI
1S/C17H18N2O6S/c1-17(2)11(16(24)25)19-13(21)10(14(19)26-17)18-12(20)9(15(22)23)8-6-4-3-5-7-8/h3-7,9-11,14H,1-2H3,(H,18,20)(H,22,23)(H,24,25)/t9?,10-,11+,14-/m1/s1
FPPNZSSZRUTDAP-UWFZAAFLSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
104.0 °F - closed cup
Punto d’infiammabilità (°C)
40 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.