A6478
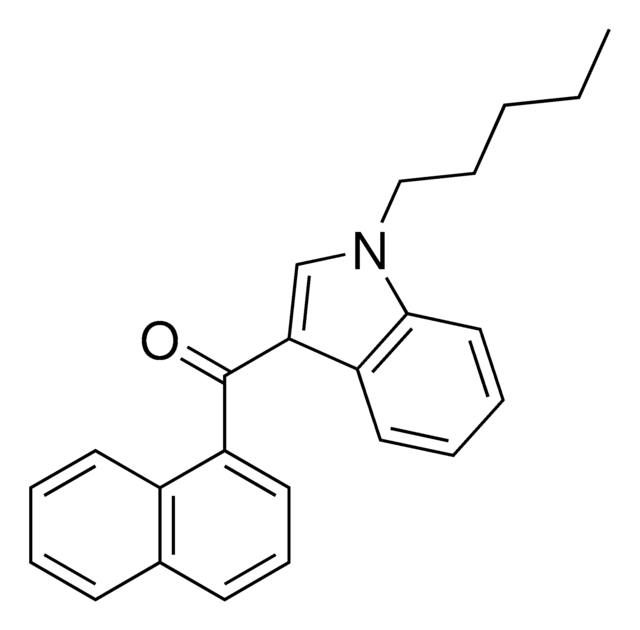
(R,S)-AM1241
≥98% (HPLC), solid
Sinonimo/i:
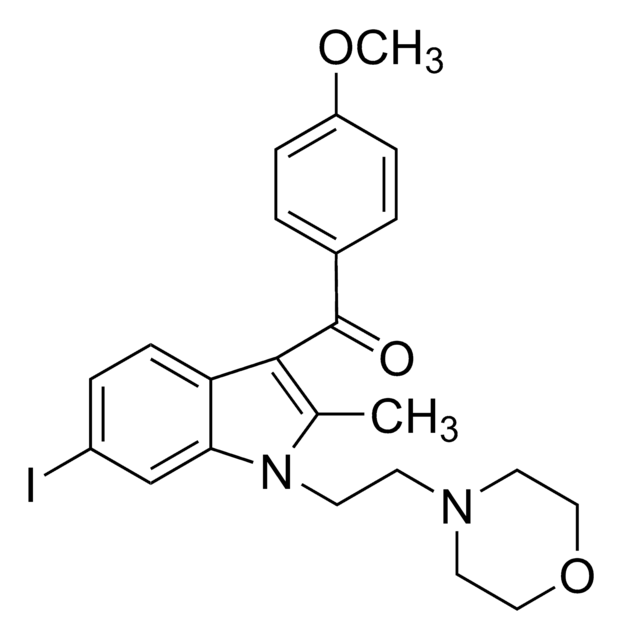
(R,S)-3-(2-Iodo-5-nitrobenzoyl)-1-(1-methyl-2-piperidinylmethyl)-1H-indole
About This Item
Prodotti consigliati
Saggio
≥98% (HPLC)
Stato
solid
Controllo stupefacenti
regulated under CDSA - not available from Sigma-Aldrich Canada
Colore
yellow
Solubilità
DMSO: ~18 mg/mL at 60 °C
Stringa SMILE
CN1CCCCC1Cn2cc(C(=O)c3cc(ccc3I)[N+]([O-])=O)c4ccccc24
InChI
1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3
ZUHIXXCLLBMBDW-UHFFFAOYSA-N
Applicazioni
- to study its inhibitory effect on bone cancer-induced pain and bone loss
- to study the effect of its interaction with 17βestradiol on proliferation activity in primary human osteoblasts
- to evaluate the sites of CB2 mediated antinociception in vivo.
Azioni biochim/fisiol
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.