A0912
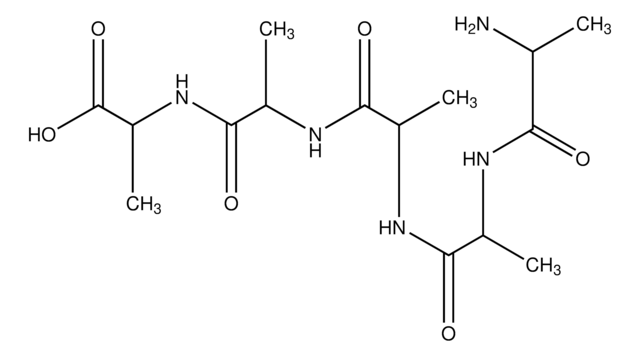
D-Ala-D-Ala
≥99%
Sinonimo/i:
D-alanyl-D-Alanine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
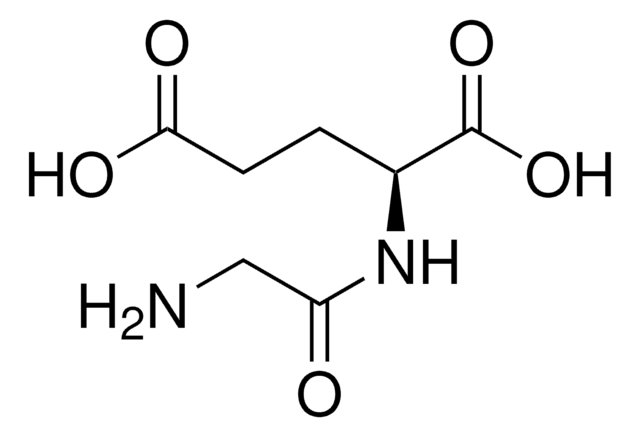
Formula empirica (notazione di Hill):
C6H12N2O3
Numero CAS:
Peso molecolare:
160.17
Numero MDL:
Codice UNSPSC:
12352202
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
D-Ala-D-Ala,
Saggio
≥99%
Stato
powder
Colore
white to off-white
Temperatura di conservazione
−20°C
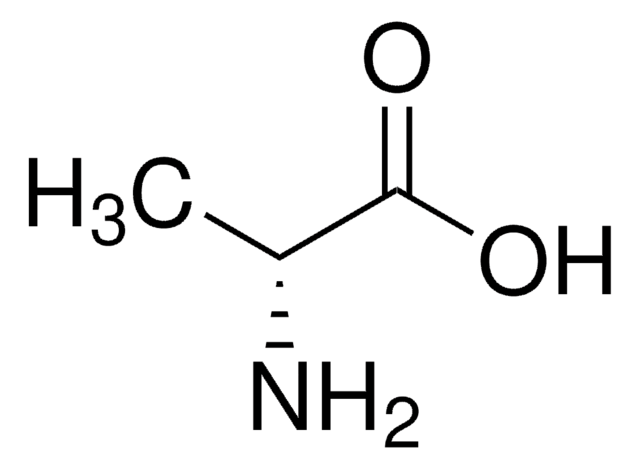
Stringa SMILE
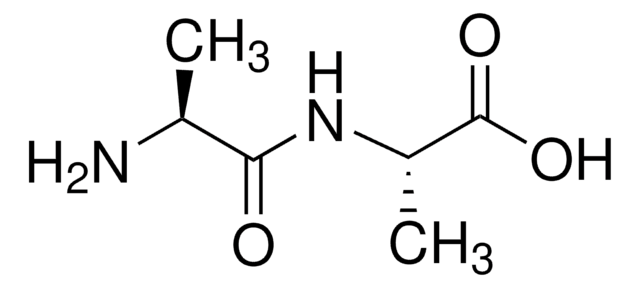
C[C@@H](N)C(=O)N[C@H](C)C(O)=O
InChI
1S/C6H12N2O3/c1-3(7)5(9)8-4(2)6(10)11/h3-4H,7H2,1-2H3,(H,8,9)(H,10,11)/t3-,4-/m1/s1
DEFJQIDDEAULHB-QWWZWVQMSA-N
Applicazioni
- Binding Mode-Based Physicochemical Screening Method Using d-Ala-d-Ala Silica Gel and Chemical Modification Approach to Facilitate Discovery of New Macrolactams, Banglactams A and B, from Nonomuraea bangladeshensis K18-0086.: Describes a novel screening method employing D-Ala-D-Ala silica gel to discover new macrolactams with potential antibacterial properties. This technique aids in identifying compounds that inhibit bacterial cell wall synthesis (Kimishima et al., 2024).
Azioni biochim/fisiol
D-Ala-D-Ala is found in the stem termini of peptidoglycan side-chain pentapeptide found in the cell walls of gram positive bacteria. The D-ala-d-ala stem termini is the site of interaction of glycopeptide antibiotics such as vancomycin and teicoplanin. D-ala-D-ala is a substrate used to study kinetics of UDPMurNAc-tripeptide D-alanyl-D-alanine-adding (ligase) enzyme.
D-Ala-D-Ala, a terminus moiety of bacterial peptidoglycans, is used for affinity chromatography and binding mechanism studies of antibiotics such as teicoplanin, ristocetin, vancomycin.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yoshiaki Kitamura et al.
Acta crystallographica. Section D, Biological crystallography, 65(Pt 10), 1098-1106 (2009-09-23)
D-Alanine-D-alanine ligase (Ddl) is one of the key enzymes in peptidoglycan biosynthesis and is an important target for drug discovery. The enzyme catalyzes the condensation of two D-Ala molecules using ATP to produce D-Ala-D-Ala, which is the terminal peptide of
Maulik N Thaker et al.
Antimicrobial agents and chemotherapy, 59(3), 1405-1410 (2014-12-17)
Vancomycin-resistant enterococci (VRE) are notorious clinical pathogens restricting the use of glycopeptide antibiotics in the clinic setting. Routine surveillance to detect VRE isolated from patients relies on PCR bioassays and chromogenic agar-based test methods. In recent years, we and others
Gareth A Prosser et al.
Antimicrobial agents and chemotherapy, 60(10), 6091-6099 (2016-08-03)
The increasing global prevalence of drug resistance among many leading human pathogens necessitates both the development of antibiotics with novel mechanisms of action and a better understanding of the physiological activities of preexisting clinically effective drugs. Inhibition of peptidoglycan (PG)
I Tytgat et al.
Current medicinal chemistry, 16(20), 2566-2580 (2009-07-16)
DD-ligases catalyze the synthesis of the D-Ala-D-Ala and D-Ala-D-Ser dipeptides or the D Ala-D-Lac depsipeptide in an early step of peptidoglycan synthesis. Their function is essential for bacterial growth and specific to bacteria, making them attractive targets for the development
Ivona Pavkova et al.
Frontiers in cellular and infection microbiology, 7, 503-503 (2018-01-13)
The DsbA homolog of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.