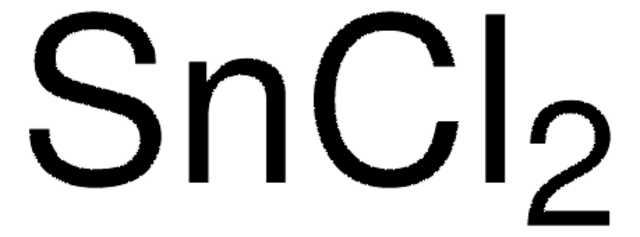96527
Tin(II) chloride dihydrate
suitable for AAS
Sinonimo/i:
Stannous chloride dihydrate
About This Item
Prodotti consigliati
Saggio
≥96.0% (RT)
Livello qualitativo
Stato
crystals
tecniche
AAS: suitable
P. ebollizione
652 °C (lit.)
Punto di fusione
37-38 °C (dec.) (lit.)
Solubilità
hydrochloric acid: passes test
Anioni in tracce
sulfate (SO42-): ≤20 mg/kg
Cationi in tracce
As: ≤1 mg/kg
Ca: ≤50 mg/kg
Cu: ≤10 mg/kg
Fe: ≤20 mg/kg
Hg: ≤5 μg/kg
K: ≤50 mg/kg
NH4+: ≤10 mg/kg
Na: ≤100 mg/kg
Pb: ≤50 mg/kg
Stringa SMILE
O.O.Cl[SnH2]Cl
InChI
1S/2ClH.2H2O.Sn/h2*1H;2*1H2;/q;;;;+2/p-2
FWPIDFUJEMBDLS-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- As a reducing agent for the determination of hydride forming species by AAS.
- In the conversion of organomercurials into inorganic mercury determined using a flow injection-cold vapor atomic absorption spectrometry
- In the determination of total mercury content, using atomic fluorescence spectroscopy and mercury speciation was performed using gas chromatography inductively coupled plasma mass spectrometry (GC-ICPMS).
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 Oral - STOT SE 3
Organi bersaglio
Cardio-vascular system, Respiratory system
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.