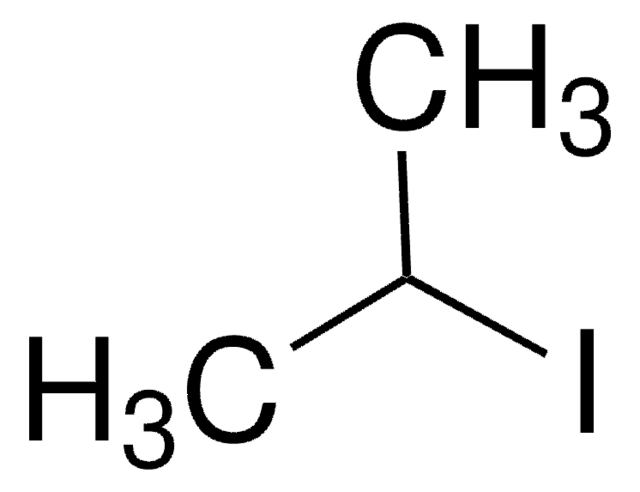
456756
Iodomethane solution
2.0 M in tert-butyl methyl ether, contains copper as stabilizer
Sinonimo/i:
Methyl iodide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
CH3I
Numero CAS:
Peso molecolare:
141.94
Beilstein:
969135
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Tensione di vapore
16.85 psi ( 55 °C)
4.86 psi ( 20 °C)
Livello qualitativo
contiene
copper as stabilizer
Concentrazione
2.0 M in tert-butyl methyl ether
P. ebollizione
41-43 °C
Densità
0.933 g/mL at 25 °C
Gruppo funzionale
alkyl halide
iodo
Temperatura di conservazione
2-8°C
Stringa SMILE
CI
InChI
1S/CH3I/c1-2/h1H3
INQOMBQAUSQDDS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
The product is 2M solution of iodomethane in tert-butyl methyl ether. Iodomethane also known as methyl iodide is an alkyl halide commonly employed as methylating agent.
Applicazioni
- 8-plex LC-MS/MS Analysis of Permethylated N-Glycans Achieved by Using Stable Isotopic Iodomethane.: This research demonstrates the use of iodomethane in stable isotope labeling for the mass spectrometric analysis of permethylated N-glycans. The method enhances the accuracy and sensitivity of glycomic studies (Dong et al., 2019).
- Comparative glycomic profiling of isotopically permethylated N-glycans by liquid chromatography/electrospray ionization mass spectrometry.: The study presents a comparative analysis of N-glycan profiles using isotopically labeled iodomethane, improving the resolution and identification of glycan structures in complex biological samples (Hu et al., 2013).
- Antifungal property of quaternized chitosan and its derivatives.: This paper explores the synthesis of quaternized chitosan derivatives using iodomethane and their subsequent antifungal activities. The results suggest potential applications in biomedical and agricultural fields (Sajomsang et al., 2012).
- Validation of two fluoro-analogues of N,N-dimethyl-2-(2′-amino-4′-hydroxymethyl-phenylthio)benzylamine as serotonin transporter imaging agents using microPET.: This research validates fluoro-analogues of a serotonin transporter imaging agent, synthesized using iodomethane, highlighting its potential in neuroimaging applications (Jarkas et al., 2010).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
-0.4 °F
Punto d’infiammabilità (°C)
-18 °C
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Selective mono-methylation of arylacetonitriles and methyl arylacetates by dimethyl carbonate.
Selva M, et al.
Journal of the Chemical Society. Perkin Transactions 1, 10, 1323-1328 (1994)
Xiao-Dan Shi et al.
Food chemistry, 271, 338-344 (2018-09-22)
Dictyophora echinovolvata is a kind of edible mushroom in the Dictyophora genus, of which polysaccharide is an important chemical substance. Herein, three polysaccharide fractions (DEP-4P, DEP-6P and DEP-8P) were prepared from water extract of D. echinovolvata using gradient ethanol precipitation
Microhydration effects on the intermediates of the S(N)2 reaction of iodide anion with methyl iodide.
Keisuke Doi et al.
Angewandte Chemie (International ed. in English), 52(16), 4380-4383 (2013-01-31)
Kazunori Kawamura et al.
Nuclear medicine and biology, 39(1), 89-99 (2011-08-13)
To explore the possible use of positron emission tomography (PET) probes for imaging of I(2)-imidazoline receptors (I(2)Rs) in peripheral tissues, we labeled two new I(2)R ligands, 2-[2-(o-tolyl)vinyl]-4,5-dihydro-1H-imidazole (K(i) for I(2)Rs, 3.7 nM) and 2-[2-(o-tolyl)ethyl]-4,5-dihydro-1H-imidazole (K(i) for I(2)Rs, 1.7 nM) with
Warayuth Sajomsang et al.
International journal of biological macromolecules, 50(1), 263-269 (2011-11-22)
Five water-soluble chitosan derivatives were carried out by quaternizing either iodomethane or N-(3-chloro-2-hydroxypropyl) trimethylammonium chloride (Quat188) as a quaternizing agent under basic condition. The degree of quaternization (DQ) ranged between 28±2% and 90±2%. The antifungal activity was evaluated by using
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.