T87807
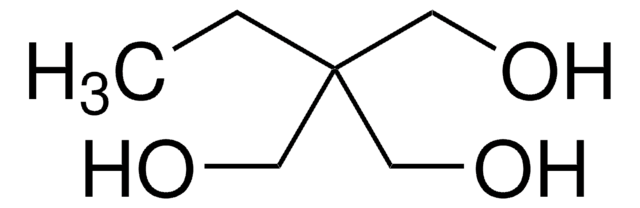
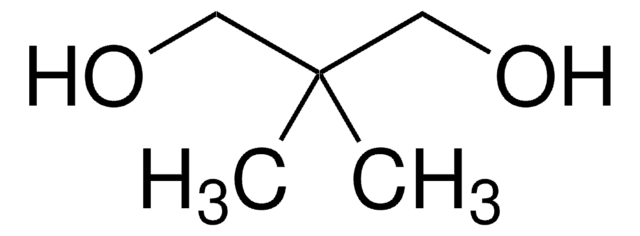
1,1,1-Tris(hydroxymethyl)ethane
≥98%
Sinonimo/i:
2-Hydroxymethyl-2-methyl-1,3-propanediol, Trimethylolethane
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3C(CH2OH)3
Numero CAS:
Peso molecolare:
120.15
Beilstein:
1304452
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥98%
Punto di fusione
193-195 °C (lit.)
Stringa SMILE
CC(CO)(CO)CO
InChI
1S/C5H12O3/c1-5(2-6,3-7)4-8/h6-8H,2-4H2,1H3
QXJQHYBHAIHNGG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
1,1,1-Tris(hydroxymethyl)ethane can be used as a reactant to synthesize:
It can also be used as a tridentate O-donor ligand in the:
- Hyperbranched polyesters by polycondensation reaction with dimethyl esters of aliphatic dicarboxylic acids.
- Trimeric anionic surfactants by reacting with long-chain α-bromo fatty acids.
It can also be used as a tridentate O-donor ligand in the:
- Copper-catalyzed cross-coupling reactions between aryl iodides and amides, thiols, and phenols to prepare corresponding products via formation of C−N, C−S, and C−O bonds.
- N-arylation of azaheterocycles with aryl iodides using copper-catalyst.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
320.0 °F - closed cup
Punto d’infiammabilità (°C)
160.00 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Highly Efficient CuI-Catalyzed N-Arylation of Azaheterocycles with Aryl Iodides Using 1, 1, 1-Tris (Hydroxymethyl) ethane as a Tridentate O-Donor Ligand: A Shorter Route to Toloxatone and Formal Synthesis of Linezolid
Chen H-H, et al.
J. Chin. Chem. Soc., 57(1), 14-18 (2010)
Synthesis and surface-active properties of a homologous series of star-like triple-chain anionic surfactants derived from 1, 1, 1-tris (hydroxymethyl) ethane
Li Xu, et al.
Journal of Surfactants and Detergents, 19(1), 129-135 (2016)
Eunmi Hong et al.
International journal of pharmaceutics, 574, 118893-118893 (2019-11-26)
Combination therapy, a treatment regimen that combines more than two therapeutic agents to diseased tissues has recently gained increasing attentions in anticancer therapy. As cancer cells are more vulnerable to oxidative stress and heat compared to normal cells, we developed
1, 1, 1-Tris (hydroxymethyl) ethane as a new, efficient, and versatile tripod ligand for copper-catalyzed cross-coupling reactions of aryl iodides with amides, thiols, and phenols
Chen Y-J and Chen H-H
Organic Letters, 8(24), 5609-5612 (2006)
Ningqiang Gong et al.
Nature nanotechnology, 14(4), 379-387 (2019-02-20)
Mitochondrial redox homeostasis, the balance between reactive oxygen species and antioxidants such as glutathione, plays critical roles in many biological processes, including biosynthesis and apoptosis, and thus is a potential target for cancer treatment. Here, we report a mitochondrial oxidative
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.