718750
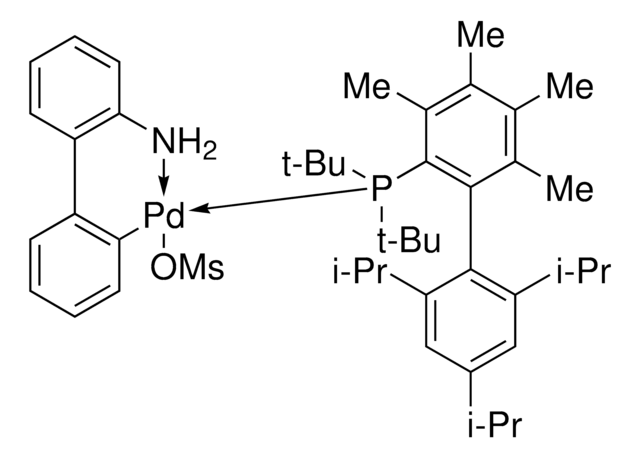
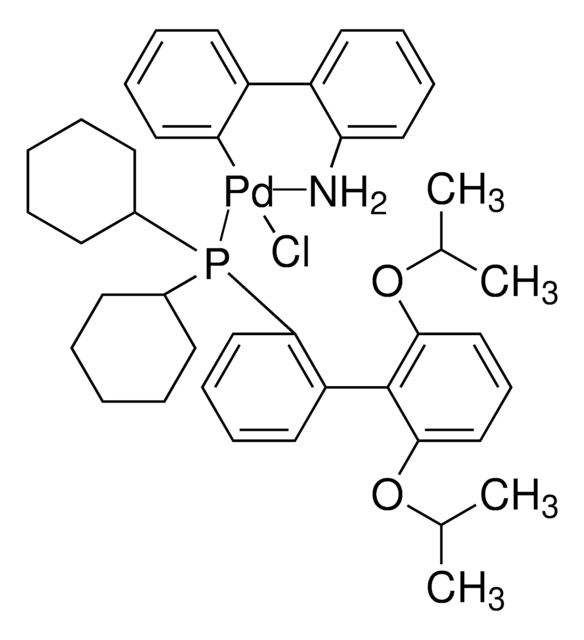
BrettPhos Pd G1, Methyl t-Butyl Ether Adduct
may contain up to 1 mole equivalent of MTBE, 97%
Sinonimo/i:
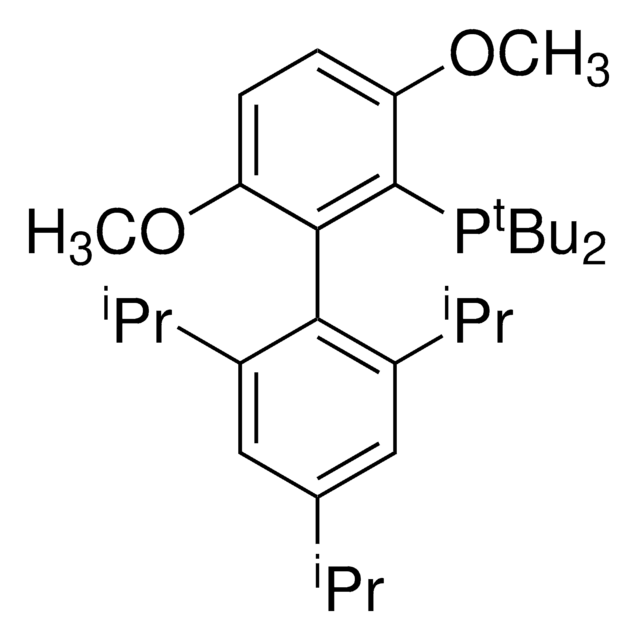
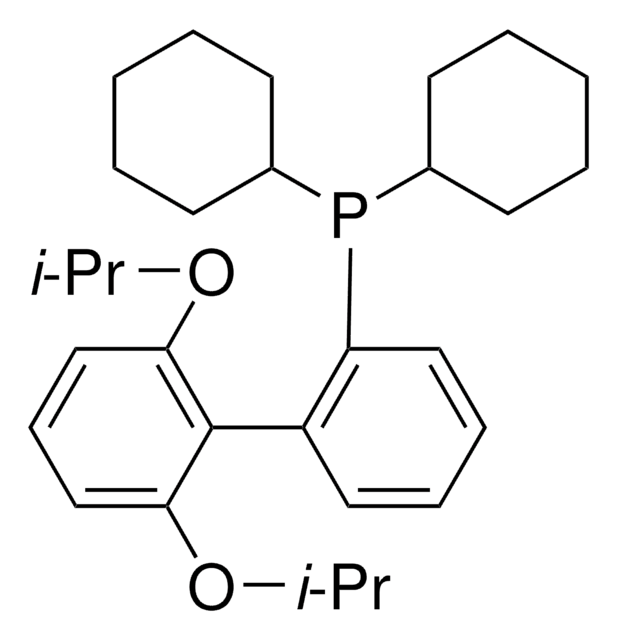
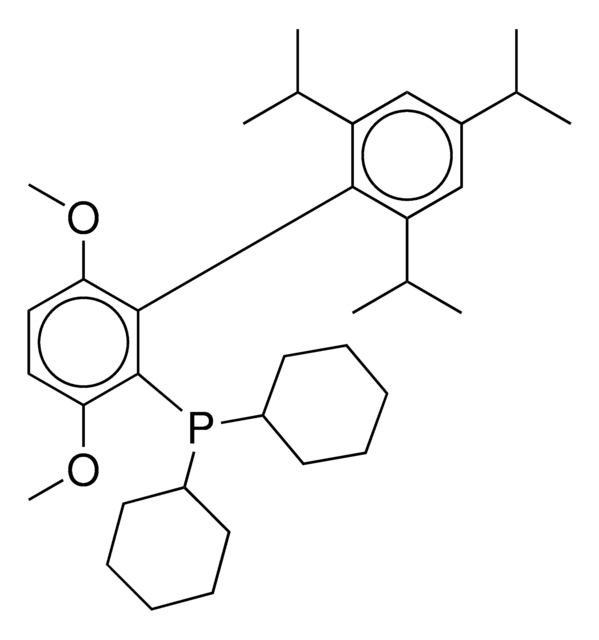
(BrettPhos) palladium(II) phenethylamine chloride, BrettPhos Palladacycle, BrettPhos precatalyst, Chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2′,4′, 6′-triisopropyl-1,1′-biphenyl][2-(2-aminoethyl)phenyl]palladium(II)
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Forma fisica
solid
Caratteristiche
generation 1
Impiego in reazioni chimiche
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
Punto di fusione
198-204 °C
Gruppo funzionale
phosphine
Stringa SMILE
COC(C)(C)C.NCCc1ccccc1[Pd]Cl.COc2ccc(OC)c(c2P(C3CCCCC3)C4CCCCC4)-c5c(cc(cc5C(C)C)C(C)C)C(C)C
InChI
1S/C35H53O2P.C8H10N.C5H12O.ClH.Pd/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28;9-7-6-8-4-2-1-3-5-8;1-5(2,3)6-4;;/h19-25,27-28H,9-18H2,1-8H3;1-4H,6-7,9H2;1-4H3;1H;/q;;;;+1/p-1
OWHWOTGYDWMPCA-UHFFFAOYSA-M
Descrizione generale
Applicazioni
Catalyst for:
- C,N-cross coupling of unprotected 3-halo-2-aminopyridines with primary and secondary amines
- Amination reaction
- N-arylation of aminophenols
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.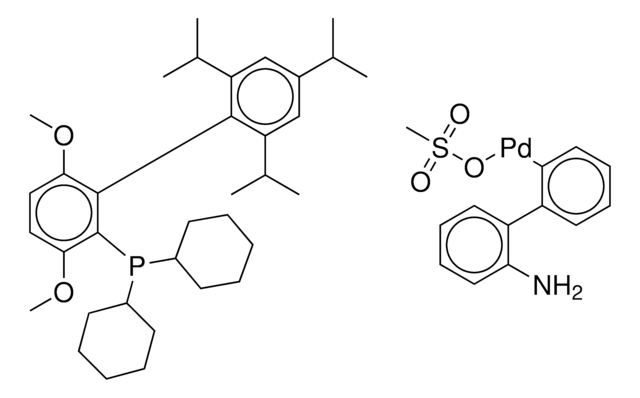

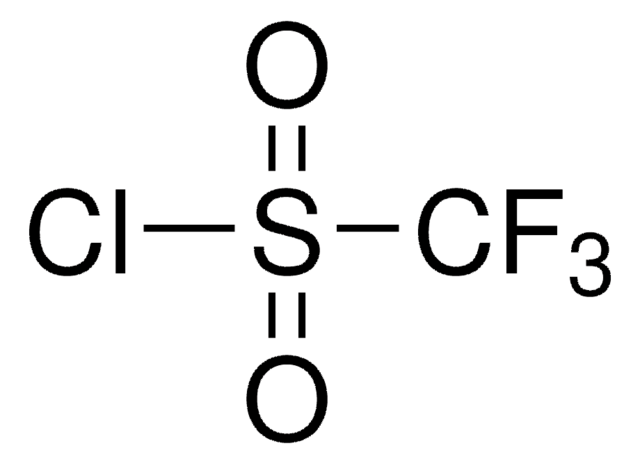
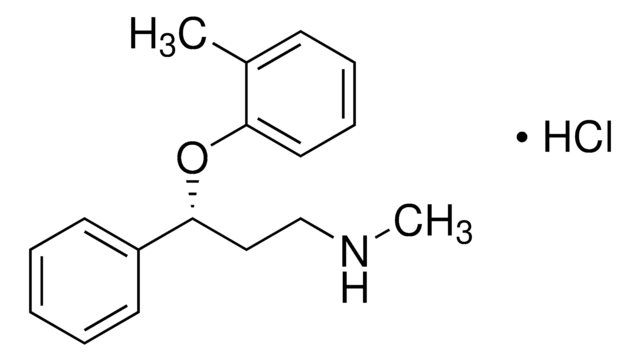
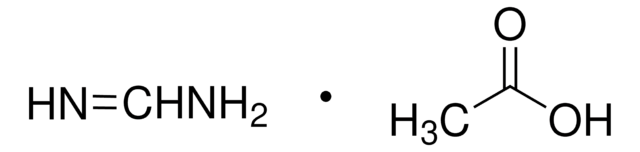
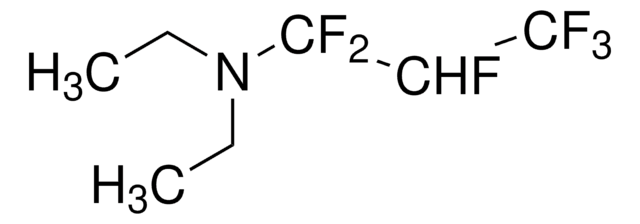
 95%](/deepweb/assets/sigmaaldrich/product/structures/151/609/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1/640/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1.png)