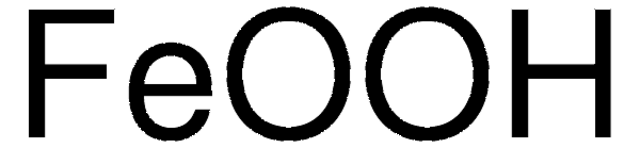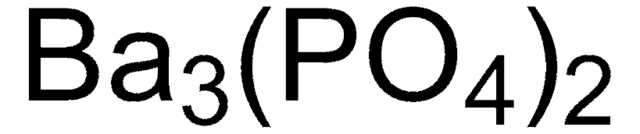71063
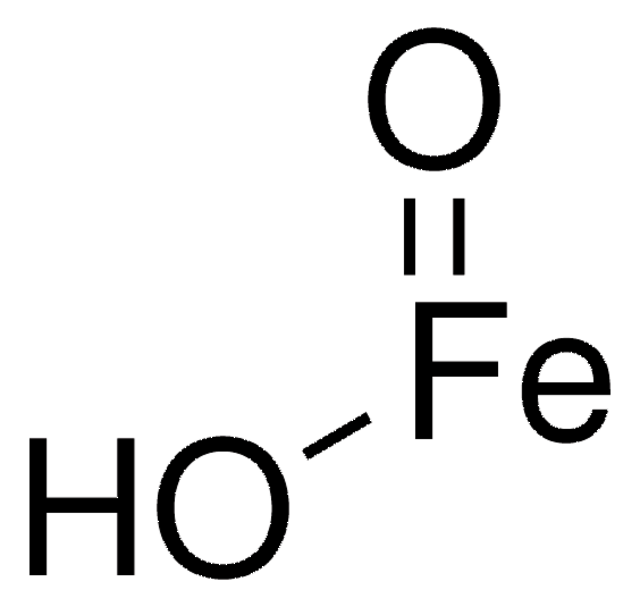
Goethite
30-63% Fe
Sinonimo/i:
Iron(III) oxide, Ferric hydroxide oxide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
Fe(OH)O
Numero CAS:
Peso molecolare:
88.85
Numero CE:
Numero MDL:
Codice UNSPSC:
12352300
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Stato
powder
Livello qualitativo
Concentrazione
30-63% Fe
Stringa SMILE
O[Fe]=O
InChI
1S/Fe.H2O.O/h;1H2;/q+1;;/p-1
AEIXRCIKZIZYPM-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
contains varying amounts of MgO2, SiO2, CaO, Al2O3
Applicazioni
adsorbent
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Johannes Kulenkampff et al.
Scientific reports, 8(1), 7091-7091 (2018-05-08)
Phenoxyalkanoic acids like the 4-chloro-2-methylphenoxyacetic acid (MCPA) are the second highest used xenobiotic herbicides worldwide after glyphosate because of their apparently favorable environmental properties. Experimental batch equilibration data suggested a reduced Cu adsorption efficiency with the soil mineral goethite below
Wenting Ma et al.
NPJ biofilms and microbiomes, 3, 4-4 (2017-06-27)
Clay minerals and metal oxides, as important parts of the soil matrix, play crucial roles in the development of microbial communities. However, the mechanism underlying such a process, particularly on the formation of soil biofilm, remains poorly understood. Here, we
Tao Wang et al.
The New phytologist, 228(2), 697-711 (2020-04-13)
In nitrogen (N)-limited boreal forests, trees depend on the decomposing activity of their ectomycorrhizal (ECM) fungal symbionts to access soil N. A large fraction of this N exists as proteinaceous compounds associated with mineral particles. However, it is not known
Adele M Jones et al.
Environmental science & technology, 51(21), 12573-12582 (2017-10-05)
In this study, temporal changes in the redox properties of three 0.5 g/L smectite suspensions were investigated-a montmorillonite (MAu-1) and two nontronites (NAu-1 and NAu-2) in the presence of 1 mM aqueous Fe(II) at pH 7.8. X-ray absorption spectroscopy revealed
Michael P Schmidt et al.
Langmuir : the ACS journal of surfaces and colloids, 33(34), 8525-8532 (2017-07-22)
DNA fate in soil plays an important role in the cycling of genetic information in the environment. Adsorption onto mineral surfaces has great impact on this function. This study probes the kinetics, equilibrium behavior and bonding mechanisms associated with adsorption
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.