560529
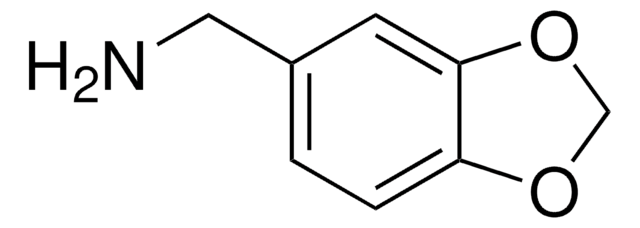
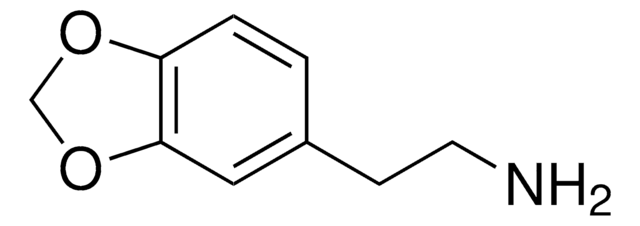
3,4-Methylenedioxyphenethylamine hydrochloride
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H11NO2 · HCl
Numero CAS:
Peso molecolare:
201.65
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
216-218 °C (lit.)
Gruppo funzionale
amine
Stringa SMILE
Cl.NCCc1ccc2OCOc2c1
InChI
1S/C9H11NO2.ClH/c10-4-3-7-1-2-8-9(5-7)12-6-11-8;/h1-2,5H,3-4,6,10H2;1H
NDYXFQODWGEGNU-UHFFFAOYSA-N
Descrizione generale
3,4-Methylenedioxyphenethylamine hydrochloride can be synthesized by reacting aluminum chloride, LiAlH4 and 3,4-methylenedioxyphenylacetonitrile.
Applicazioni
3,4-Methylenedioxyphenethylamine hydrochloride may be used to synthesize N-(3,4-methylenedioxyphenethyl)-2-(3-bromo-4-methoxyphenyl)acetamide.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Kjell A Mortier et al.
Rapid communications in mass spectrometry : RCM, 16(9), 865-870 (2002-04-12)
Paramethoxyamphetamine (PMA) is an amphetamine-like designer drug that has emerged recently on the European illicit drug market. This drug has a wicked reputation, as a number of lethal intoxications have occurred. A method using high-performance liquid chromatography coupled to ion
Laura Aalberg et al.
Journal of chromatographic science, 41(5), 227-233 (2003-07-05)
Three regioisomeric 3,4-methylenedioxyphenethylamines having the same molecular weight and major mass spectral fragments of equivalent mass have been reported as components of clandestine drug samples in recent years. These drugs of abuse are 3,4-methylenedioxy-N-ethylamphetamine, 3,4-methylenedioxy-N,N-dimethylamphetamine, and N-methyl-1-(3,4-methylenedioxyphenyl)-2-butanamine. These three compounds
Jan G Bruhn et al.
Journal of psychoactive drugs, 40(2), 219-222 (2008-08-30)
Human interest in psychoactive phenethylamines is known from the use of mescaline-containing cacti and designer drugs such as Ecstasy. From the alkaloid composition of cacti we hypothesized that substances resembling Ecstasy might occur naturally. In this article we show that
An aryne route to laureline, and related topics.
Gibson MS, et al.
J. Chem. Soc. Sect. C, 16, 2234-2238 (1970)
Jon E Sprague et al.
The Journal of pharmacology and experimental therapeutics, 305(1), 159-166 (2003-03-22)
An acute and potentially life-threatening complication associated with the recreational use of the 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) is hyperthermia. In the present study, Sprague-Dawley rats treated with MDMA (40 mg/kg s.c.) responded with a significant increase (maximal at 1 h) in
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)