538396
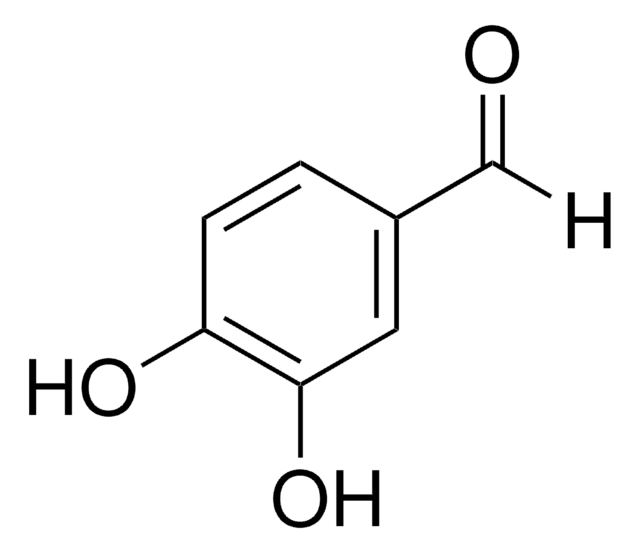
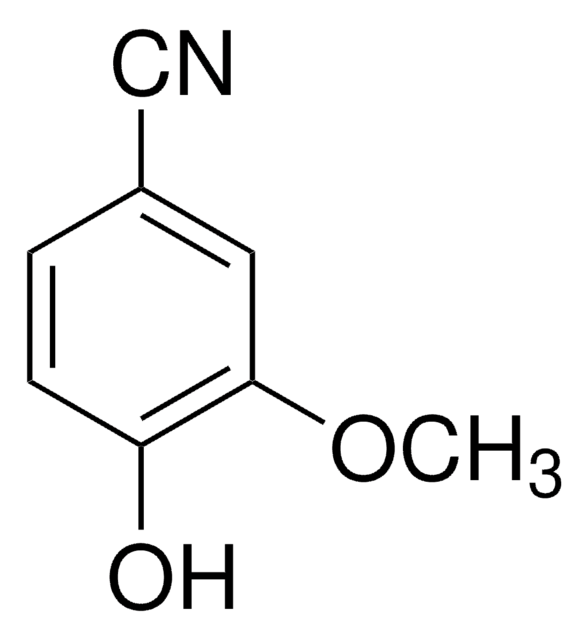
3,4-Dihydroxybenzonitrile
97%
Sinonimo/i:
Protocatechuonitrile
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(OH)2C6H3CN
Numero CAS:
Peso molecolare:
135.12
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Punto di fusione
155-159 °C (lit.)
Stringa SMILE
Oc1ccc(cc1O)C#N
InChI
1S/C7H5NO2/c8-4-5-1-2-6(9)7(10)3-5/h1-3,9-10H
NUWHYWYSMAPBHK-UHFFFAOYSA-N
Descrizione generale
3,4-Dihydroxybenzonitrile can be prepared from 4-hydroxy-3-methoxybenzonitrile. It can also be synthesized by reacting 3,4-dimethoxybenzonitrile, lithium diisopropylamide (LDA) and 1,3-dimethyl-2-imidazolidinone (DMEU).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
M J Nelson et al.
Biochemistry, 34(46), 15219-15229 (1995-11-21)
Ferric soybean lipoxygenase forms stable complexes with 4-substituted catechols. The structure of the complex between the enzyme and 3,4-dihydroxybenzonitrile has been studied by resonance Raman, electron paramagnetic resonance, visible, and X-ray spectroscopies. It is a bidentate iron-catecholate complex with at
M M Wick et al.
Journal of pharmaceutical sciences, 76(7), 513-515 (1987-07-01)
This report describes a structure-activity analysis of isomers of three classes of dihydroxybenzene derivatives, including dihydroxybenzaldoxime, dihydroxybenzaldehyde, and dihydroxybenzonitrile. These derivatives were examined for their effect on ribonucleotide reductase activity, macromolecular synthesis, cell growth, and in vivo antitumor activity against
Sodium Bis (trimethylsilyl) amide and Lithium Diisopropylamide in Deprotection of Alkyl Aryl Ethers: a-Effect of Silicon
Hwu JR, et al.
The Journal of Organic Chemistry, 62.12 , 4097-4104 (1997)
The synthetic technology of 3, 4-dihydroxybenzonitrile
WEI HW, et al.
Fine and Specialty Chemicals / Jing Xi Yu Zhuan Yong Hua Xue Pin, 9, 012-012 (2011)
The Reactivity and Reaction Pathway of Fenton Reactions Driven by Substituted 1,2-Dihydroxybenzenes.
Pablo Salgado et al.
Environmental science & technology, 51(7), 3687-3693 (2017-03-09)
Fenton systems are interesting alternatives to advanced oxidation processes (AOPs) applied in soil or water remediation. 1,2-Dihydroxybenzenes (1,2-DHBs) are able to amplify the reactivity of Fenton systems and have been extensively studied in biological systems and for AOP applications. To
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.