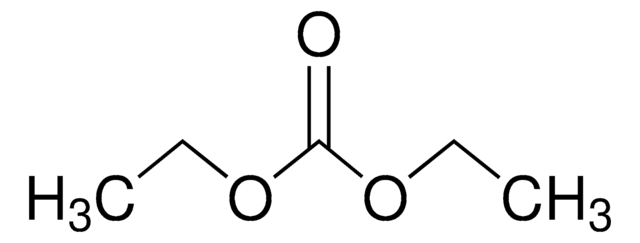
517135
Diethyl carbonate
anhydrous, ≥99%
Sinonimo/i:
Diatol, Eufin, H-DEC
About This Item
Prodotti consigliati
Grado
anhydrous
Livello qualitativo
Densità del vapore
4.1 (vs air)
Tensione di vapore
10 mmHg ( 23.8 °C)
59 mmHg ( 37.8 °C)
Saggio
≥99%
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
<0.002% water
<0.005% water (100 mL)
Indice di rifrazione
n20/D 1.384 (lit.)
P. ebollizione
126-128 °C (lit.)
Punto di fusione
−43 °C (lit.)
Solubilità
water: insoluble
Densità
0.975 g/mL at 25 °C (lit.)
Gruppo funzionale
carbonate
Categoria alternativa più verde
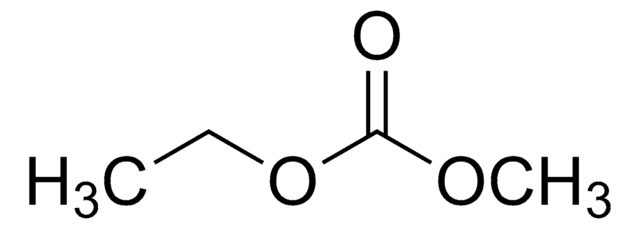
Stringa SMILE
O=C(OCC)OCC
InChI
1S/C5H10O3/c1-3-7-5(6)8-4-2/h3-4H2,1-2H3
OIFBSDVPJOWBCH-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Synthesis of β-enamino esters.
- Synthesis of carbamates and unsymmetrical alkyl carbonates, via reaction with aliphatic amines or alcohols by using a hybrid organic-inorganic material prepared by anchoring TBD to MCM-41 silica.
- As solvent in ruthenium catalyzed direct functionalisation of arene C-H bonds by aryl halides.
- To compose the commercial liquid electrolyte for lithium ion batteries.
- Homogeneous alkoxycarbonylation of cellulose.
Caratteristiche e vantaggi
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
77.0 °F - closed cup
Punto d’infiammabilità (°C)
25 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Electrode Materials for Lithium Ion Batteries
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 517135-1L | 4061832541563 |
| 517135-100ML | 4061832541556 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.