458910
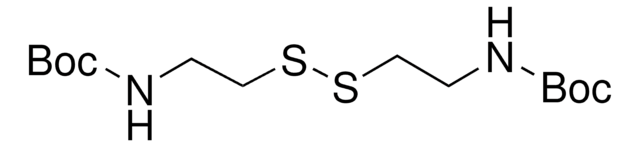
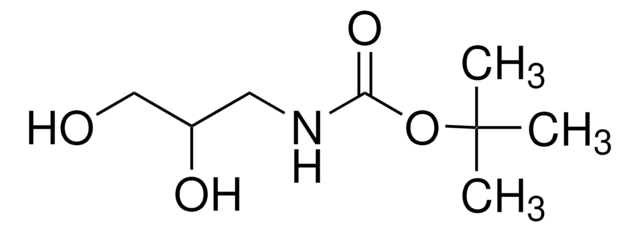
2-(Boc-amino)ethanethiol
97%
Sinonimo/i:
tert-Butyl N-(2-mercaptoethyl)carbamate
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Indice di rifrazione
n20/D 1.474 (lit.)
P. ebollizione
68 °C/0.3 mmHg (lit.)
Densità
1.049 g/mL at 20 °C (lit.)
Gruppo funzionale
Boc
amine
thiol
Stringa SMILE
SCCNC(OC(C)(C)C)=O
InChI
1S/C7H15NO2S/c1-7(2,3)10-6(9)8-4-5-11/h11H,4-5H2,1-3H3,(H,8,9)
GSJJCZSHYJNRPN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
228.2 °F - closed cup
Punto d’infiammabilità (°C)
109 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 458910-25ML | 4061838127938 |
| 458910-5ML | 4061838127945 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.