394408
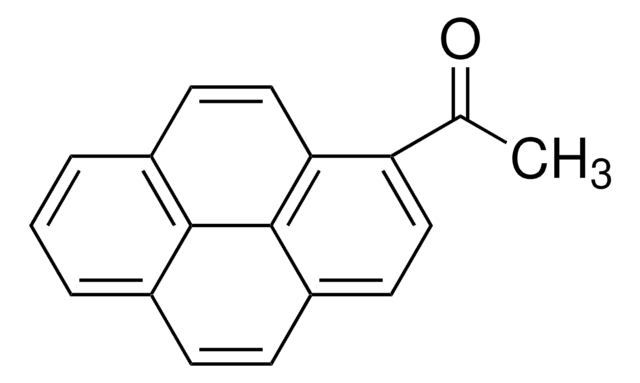
1-(Bromoacetyl)pyrene
97%
Sinonimo/i:
2-Bromo-1-(1-pyrenyl)ethanone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C18H11BrO
Numero CAS:
Peso molecolare:
323.18
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Forma fisica
solid
Punto di fusione
129-131 °C (lit.)
Stringa SMILE
BrCC(=O)c1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H11BrO/c19-10-16(20)14-8-6-13-5-4-11-2-1-3-12-7-9-15(14)18(13)17(11)12/h1-9H,10H2
KAEDEGFCOPIKKM-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
1-(Bromoacetyl)pyrene (BAP) is a pyrene derivative. It has been synthesized by reacting cupric bromide with 1-acetylpyrene. Studies suggest that the introduction of a bromoacetyl chromophoric moiety to pyrene drastically increases the photoinitiating efficiency of pyrenes.
Applicazioni
1-(Bromoacetyl)pyrene is suitable for use in the following studies:
- As an initiator in the bulk polymerization of 2-ethyl-2-oxazoline to generate pyrene labelled poly(2-ethyl-2-oxazoline) (PETOX-py).
- As a fluorophore in the generation of podand-type fluoroionophores with two pyrene moieties.
- As a fluorescent labeling agent for the determination of okadaic acid toxin by HPLC with fluorescence detection.
- As a photoremovable protecting group for carboxylic acids and amino acids.
- As a photoinitiator in the photopolymerization of styrene with methylmethacrylate.
- As a reactant in the synthesis of potentially tetradentate pyrene appended ligands.
- As a derivatizing agent of dialkyl phosphates (DAP) in the HPTLC method of quantitative determination of DAP in fruit juices.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Aquatic Chronic 4 - Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
S Kamada et al.
Journal of chromatography, 272(1), 29-41 (1983-01-14)
A fluorescence high-performance liquid chromatographic method is described for the determination of free and conjugated bile acids in serum and bile. Free and conjugated bile acids are extracted from serum or bile using a Sep-Pak C18 cartridge and then fractionated
J C González et al.
Journal of chromatography. A, 793(1), 63-70 (1998-02-20)
Okadaic acid (OA) and dinophysistoxin-2, two of the main diarrhetic shellfish toxins, can be determined by high-performance liquid chromatography coupled to fluorimetry as pyrenacyl esters. Toxin fluorescent derivatives were obtained after quantitative derivatization with 1-bromoacetylpyrene in acetonitrile. An efficient improvement
José C González et al.
Journal of agricultural and food chemistry, 50(2), 400-405 (2002-01-10)
The natural contamination of shellfish with diarrheic shellfish toxins (DSP) has important public health implications. To avoid the economic effects of toxic episodes on shellfish farmers and the related industry, research on artificial methods alternative to the natural detoxification of
Juraj Kronek et al.
Journal of materials science. Materials in medicine, 22(7), 1725-1734 (2011-05-24)
Poly(2-oxazolines) with varying alkyl chain lengths (e.g., methyl, ethyl, aryl) and molar masses have been tested for cell cytotoxicity in vitro. A standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used for the estimation of cell viability. Two monomers, 2-methyl-2-oxazoline and 2-ethyl-2-oxazoline
H Ochiai et al.
Journal of chromatography. B, Biomedical sciences and applications, 694(1), 211-217 (1997-06-20)
By using a fluorescent derivatization and column-switching technique, a highly sensitive and selective high-performance liquid chromatographic (HPLC) method has been developed for the determination of simvastatin (I, beta-hydroxy-delta-lactone form) and its active hydrolyzed metabolite (II, beta,delta-dihydroxy acid form of I)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.