286281
Indole-3-acetamide
98%
Sinonimo/i:
3-Indolylacetamide, NSC 1969
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H10N2O
Numero CAS:
Peso molecolare:
174.20
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
148-150 °C (lit.)
Gruppo funzionale
amide
Stringa SMILE
NC(=O)Cc1c[nH]c2ccccc12
InChI
1S/C10H10N2O/c11-10(13)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H2,11,13)
ZOAMBXDOGPRZLP-UHFFFAOYSA-N
Descrizione generale
Indole-3-acetamide is an auxin precursor.
Applicazioni
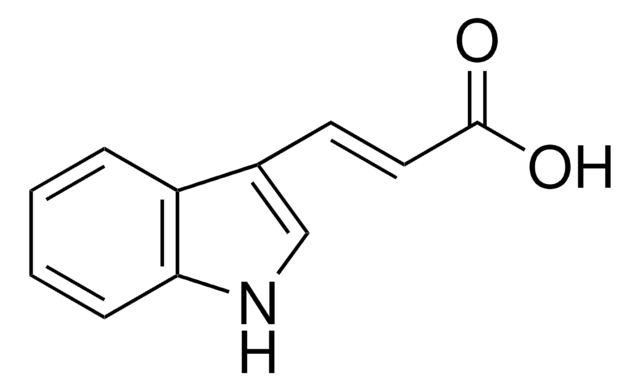
Indole-3-acetamide was used in the synthesis of [5.5.6.6]diazafenestrane skeleton and indole-3-acetic acid.
Reactant for the synthesis of:
- PET agent for imaging of protein kinase C
- A potential agent against Prion Disease
- Protein kinase C (PKC) inhibitor bisindolylmaleimide IV
- Glycogen synthase kinase-3ß (GSK-3ß) inhibitors
- Inhibitors of CaMKIId
- A VEGF inhibitor
- JAK3 inhibitors
- Inhibitors of NAD+-Dependent Histone Deacetylases
- Inhibitors of human adipocyte fatty acid-binding protein
- Cyclin-dependent kinase inhibitors
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Stephan Pollmann et al.
Phytochemistry, 70(4), 523-531 (2009-03-10)
Plants are suggested to produce their major growth promoting phytohormone, indole-3-acetic acid (IAA), via multiple redundantly operating pathways. Although great effort has been made and plenty of possible routes have been proposed based on experimental evidence, a complete pathway for
Casandra K Gutierrez et al.
Applied and environmental microbiology, 75(8), 2253-2258 (2009-02-17)
Strains of Vibrio spp. isolated from roots of the estuarine grasses Spartina alterniflora and Juncus roemerianus produce the phytohormone indole-3-acetic acid (IAA). The colorimetric Salkowski assay was used for initial screening of IAA production. Gas chromatography-mass spectroscopy (GC-MS) was then
Chuntao Yin et al.
Molecular plant-microbe interactions : MPMI, 27(3), 227-235 (2013-12-20)
The plant hormone indole-3-acetic acid (IAA) is best known as a regulator of plant growth and development but its production can also affect plant-microbe interactions. Microorganisms, including numerous plant-associated bacteria and several fungi, are also capable of producing IAA. The
Stephan Pollmann et al.
Phytochemistry, 62(3), 293-300 (2003-03-07)
Acylamidohydrolases from higher plants have not been characterized or cloned so far. AtAMI1 is the first member of this enzyme family from a higher plant and was identified in the genome of Arabidopsis thaliana based on sequence homology with the
Christian O Dimkpa et al.
Applied and environmental microbiology, 78(5), 1404-1410 (2012-01-03)
The beneficial bacterium Pseudomonas chlororaphis O6 produces indole-3-acetic acid (IAA), a plant growth regulator. However, the pathway involved in IAA production in this bacterium has not been reported. In this paper we describe the involvement of the indole-3-acetamide (IAM) pathway
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.