215880
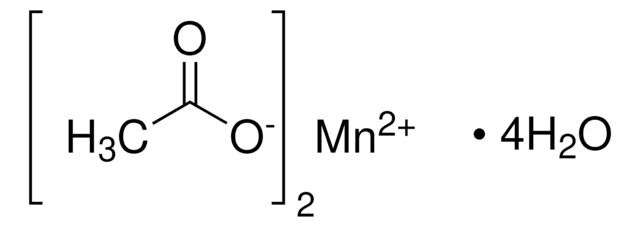
Manganese(III) acetate dihydrate
97%
Sinonimo/i:
Manganese triacetate dihydrate
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder or chunks
Impiego in reazioni chimiche
core: manganese
Stringa SMILE
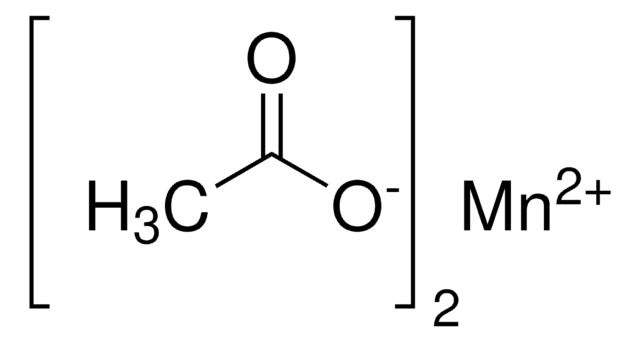
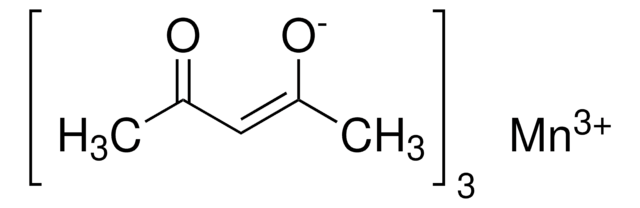
O.O.CC(=O)O[Mn](OC(C)=O)OC(C)=O
InChI
1S/3C2H4O2.Mn.2H2O/c3*1-2(3)4;;;/h3*1H3,(H,3,4);;2*1H2/q;;;+3;;/p-3
ONJSLAKTVIZUQS-UHFFFAOYSA-K
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- A precursor in the synthesis of manganese oxide (Mn3O4) nanostructures, which are employed as anode materials in lithium-ion batteries.
- A manganese source in the sol-gel synthesis of Mn-doped ZnO thin films. The incorporation of manganese ions into the ZnO lattice is essential for modifying the electronic and optical properties of the films.
- A precursor for the synthesis of manganese oxide nanoparticles using a sol-gel process. These nanoparticles are evaluated for their performance in supercapacitor applications.
- A mild and selective oxidizing agent. Catalyzes allylic oxidation of a variety of alkenes in the presence of tert-butylhydroperoxide. Reagent used for radical cyclizations and α-keto-acetoxylation.
Stato fisico
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.