167398
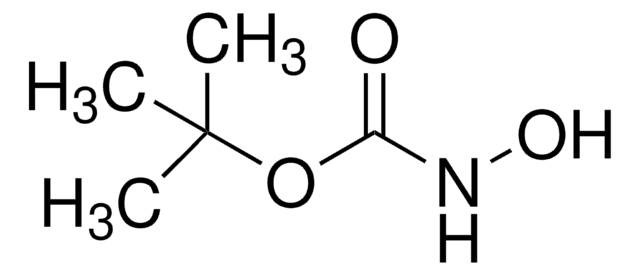
tert-Butyl carbamate
98%
Sinonimo/i:
Boc-amide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NH2COOC(CH3)3
Numero CAS:
Peso molecolare:
117.15
Beilstein:
1744500
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Forma fisica
solid
Punto di fusione
105-108 °C (lit.)
Stringa SMILE
CC(C)(C)OC(N)=O
InChI
1S/C5H11NO2/c1-5(2,3)8-4(6)7/h1-3H3,(H2,6,7)
LFKDJXLFVYVEFG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Palladium-catalyzed cross-coupling reaction of tert-butyl carbamate with various aryl(Het) halides with Cs2CO3 as base in 1,4-dioxane (solvent) has been investigated.
Applicazioni
tert-Butyl carbamate was used in palladium-catalyzed synthesis of N-Boc-protected anilines. It was used in the synthesis of tetrasubstituted pyrroles, functionalized with ester or ketone groups at C-3 position.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Maximilian Tromayer et al.
Polymer chemistry, 8(2), 451-460 (2017-03-07)
The possibility of the direct encapsulation of living cells
Pd-catalyzed amidation of aryl (Het) halides with< i> tert</i>-butyl carbamate.
Qin L, et al.
Tetrahedron Letters, 51(33), 4446-4448 (2010)
Swapna Bhagwanth et al.
The Journal of organic chemistry, 74(12), 4634-4637 (2009-06-13)
The scope of Pd-catalyzed synthesis of N-Boc-protected anilines from aryl bromides and commercially available tert-butyl carbamate is described. For the first time, this process can be conducted at room temperature (17-22 degrees C) using a combination of Pd(2)dba(3).CHCl(3) and a
One-Pot Three-Component Synthesis of Tetrasubstituted NH Pyrroles from Secondary Propargylic Alcohols, 1, 3-Dicarbonyl Compounds and tert-Butyl Carbamate.
Cadierno V, et al.
Journal of Heterocyclic Chemistry, 47(1), 233-236 (2010)
Tetsuo Cai et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(43), 8600-8610 (2019-09-19)
γ-Secretase is an intramembrane-cleaving protease that generates the toxic species of the amyloid-β peptide (Aβ) that is responsible for the pathology of Alzheimer disease. The catalytic subunit of γ-secretase is presenilin 1 (PS1), which is a polytopic membrane protein with
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.