153494
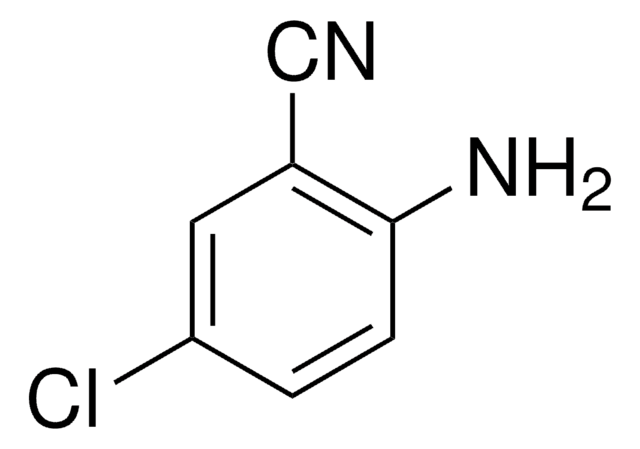
2-Amino-5-nitrobenzonitrile
95%
Sinonimo/i:
2-Cyano-4-nitroaniline, 5-Nitroanthranilonitrile
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
O2NC6H3(NH2)CN
Numero CAS:
Peso molecolare:
163.13
Beilstein:
1425714
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Stato
powder
Punto di fusione
200-207 °C (lit.)
Stringa SMILE
Nc1ccc(cc1C#N)[N+]([O-])=O
InChI
1S/C7H5N3O2/c8-4-5-3-6(10(11)12)1-2-7(5)9/h1-3H,9H2
MGCGMYPNXAFGFA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Alexis Enright et al.
The Analyst, 129(10), 975-978 (2004-10-01)
A series of eleven specially designed benzotriazole monoazo dyes for use in surface enhanced resonance Raman scattering studies are reported. Unlike previous benzotriazole dyes produced for SERRS, these dyes have been synthesised to be trifunctional in nature. The presence of
Syed Muhammad Saad et al.
European journal of medicinal chemistry, 108, 13-20 (2015-12-01)
4-Arylamino-6-nitroquinazolines (2-25) were synthesized and evaluated for their leishmanicidal activities against Leishmania major promastigotes in vitro with IC50 values = 1.87-61.48 μM. Among the twenty four synthetic derivatives, 4-[4'-(methylsulfanyl)phenyl]amino-6-nitroquinazoline (21), and 4-(2'-methoxyphenyl)amino-6-nitroquinazoline (8) showed excellent antileishmanial activities with IC50 values 1.87 ± 0.31 and 4.37 ± 0.02 μM, respectively
Jinho Lee et al.
Bioorganic & medicinal chemistry letters, 18(7), 2292-2295 (2008-03-21)
A novel series of 3,5-diaminoindazoles were prepared and found to be CDK inhibitors. Potent inhibitors against CDK1 and CDK2 were obtained by introduction of 1lambda(6)-isothiazolidine-1,1-dioxide at 5-position of indazole. Anti-proliferative activities of compounds were evaluated using EJ, HCT116, SW620, and
Anton Georgiev et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 175, 76-91 (2016-12-27)
In this paper three different "push-pull" 4-aminoazobenzene dyes have been synthesized in order to characterize their photochromic behavior in different solvents. The molecular geometry was optimized by DFT/B3LYP functional combined with the standard 6-31+G(d,p) basis set for trans (E) and
Jie Hu et al.
Chemical biology & drug design, 85(6), 672-684 (2014-10-21)
Quinazoline has been reported to exhibit multiple bioactivities. The aim of this study was to discover new quinazoline derivatives with preventive effect on lipopolysaccharide-induced acute lung injury via anti-inflammatory actions. Thirty-three 4-amino quinazolin derivatives were synthesized and screened for anti-inflammatory
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 153494-5G | |
| 153494-100G | 4061838740861 |
| 153494-500G |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.