145688
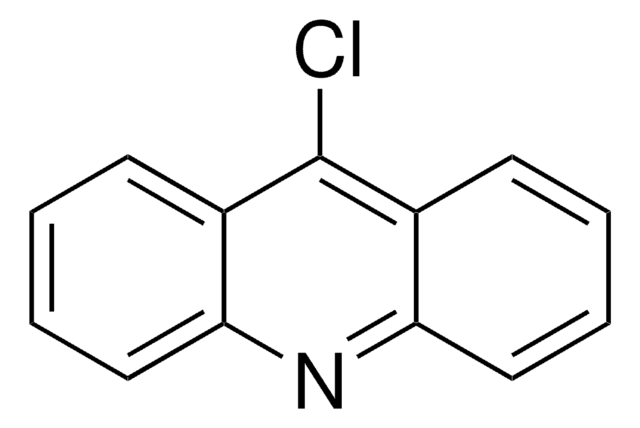
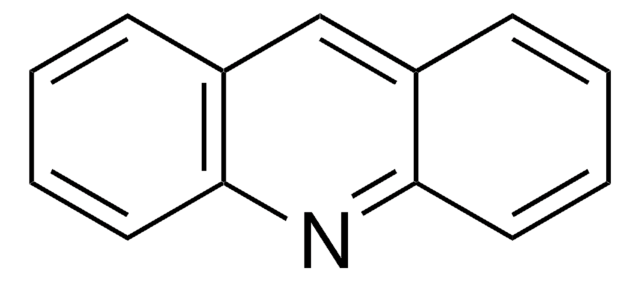
6,9-Dichloro-2-methoxyacridine
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C14H9Cl2NO
Numero CAS:
Peso molecolare:
278.13
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
163-165 °C (lit.)
Gruppo funzionale
chloro
Stringa SMILE
COc1ccc2nc3cc(Cl)ccc3c(Cl)c2c1
InChI
1S/C14H9Cl2NO/c1-18-9-3-5-12-11(7-9)14(16)10-4-2-8(15)6-13(10)17-12/h2-7H,1H3
RYRNQWYNHLLOGX-UHFFFAOYSA-N
Descrizione generale
6,9-Dichloro-2-methoxyacridine on reaction with quinolizidinylalkylamines yields 4-aminoquinoline and 9-aminoacridine derivatives.
Applicazioni
6,9-Dichloro-2-methoxyacridine was used in the synthesis of 9-amino-6-chloro-2-methoxyacridine, N′-(6-Chloro-2-methoxy-acridin-9-yl)-heptylamine and N,N′-bis-(6-chloro-2-methoxy-acridin-9-yl)-hexane-1,6-diamine.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Origin of the complex fluorescence emission of 9-amino-6-chloro-2-methoxyacridine. 1. Experiment.
Fan P, et al.
The Journal of Physical Chemistry, 93(18), 6615-6622 (1989)
C Boido Canu et al.
Bollettino chimico farmaceutico, 128(6), 212-215 (1989-06-01)
By reacting three quinolizidinylalkylamines with 4,7-dichloroquinoline and 6,9-dichloro-2-methoxyacridine six derivatives of 4-aminoquinoline and 9-aminoacridine were obtained. These compounds, which are of interest as potential antibacterial, antiprotozoarian, anti-helminthic and antitumoral agents, so far have been tested against lymphocytic leukemia P 388
Lucie Guetzoyan et al.
Bioorganic & medicinal chemistry, 17(23), 8032-8039 (2009-11-03)
A series of acridine derivatives were synthesised and their in vitro antimalarial activity was evaluated against one chloroquine-susceptible strain (3D7) and three chloroquine-resistant strains (W2, Bre1 and FCR3) of Plasmodium falciparum. Structure-activity relationship showed that two positives charges as well
Ana Gomes et al.
ChemMedChem, 9(2), 305-310 (2014-01-30)
Plasmodium falciparum, the causative agent of the most lethal form of malaria, is becoming increasingly resistant to most available drugs. A convenient approach to combat parasite resistance is the development of analogues of classical antimalarial agents, appropriately modified in order
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.