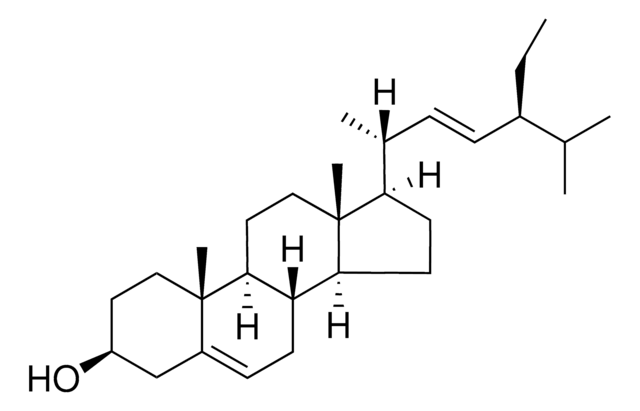
PHL89514
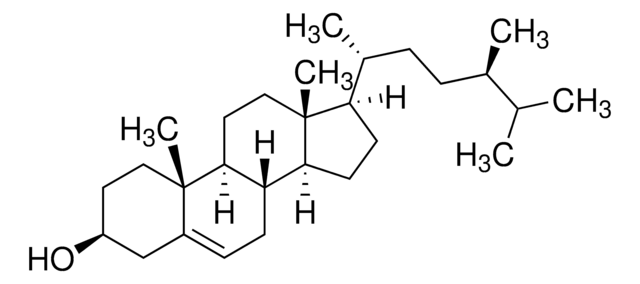
Campesterol
phyproof® Reference Substance
Synonym(s):
24α-Methyl-5-cholesten-3β-ol, 24(R)-Ergost-5-en-3β-ol
About This Item
Recommended Products
biological source
Glycine max (soybean)
grade
primary reference standard
product line
phyproof® Reference Substance
Assay
≥90.0% (GC)
form
crystals
manufacturer/tradename
PhytoLab
storage temp.
2-8°C
SMILES string
[H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)[C@@]1([H])CC[C@]4(C)[C@H](CC[C@@]24[H])[C@H](C)CC[C@H](C)C(C)C
InChI
1S/C28H48O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h9,18-20,22-26,29H,7-8,10-17H2,1-6H3/t19-,20+,22-,23-,24+,25-,26-,27-,28+/m0/s1
InChI key
SGNBVLSWZMBQTH-ZRUUVFCLSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Campesterol is a major phytosterol having a core skeleton similar to cholesterol but with a methyl side chain at C24. It is used in the management of hypercholesterolemia, besides having antioxidant, anti-inflammatory, and anti-cancer properties.
Application
- Development of two high-performance liquid chromatography (HPLC)-diode array ultraviolet/visible detection (DAD-UV/VIS) and liquid chromatography-mass spectrometry (LC-MS) methods to determine campesterol, stigmasterol, and daucosterol from the herbal samples of Artemisia apiacea
- Multi-residue determination of β-sitosterol, campesterol, and stigmasterol in rat plasma samples by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (LC-APCI-MS/MS) method
- Ultrasound-assisted extraction (UAE) of campesterol, β-sitosterol, and stigmasterol from passion fruit seed oil samples for their determination by gas chromatography-flame ionization detection (GC-FID)
- Analysis of 119 vegetable oil samples from 7 different varieties to detect and determine squalene and four sterols using a developed and validated non-destructive method based on proton nuclear magnetic resonance (1H NMR) spectroscopy combined with partial least squares (PLS) method
- Multi-residue analysis of isoquercitrin, campesterol, emodin 8-O-β-D-glucopyranoside, and quercetin in dried stem and flower extracts of Reynoutria sachalinensis by high-performance liquid chromatography coupled with diode-array detection
Other Notes
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| PHL89514-10MG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service