T7309
Trypsin from bovine pancreas
≥2,500 USP units/mg solid, meets USP testing specifications
Synonym(s):
Serine Protease 1
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
Agency
USP/NF
meets USP testing specifications
Quality Level
form
solid
specific activity
≥2,500 USP units/mg solid
mol wt
23.8 kDa
purified by
crystallization
solubility
H2O: soluble
saline: soluble
application(s)
diagnostic assay manufacturing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
For trypsin digestion of peptides, use a ratio of about 1:100 to 1:20 for trypsin:peptide. The typical use for this product is in removing adherent cells from a culture surface. The concentration of trypsin necessary to dislodge cells from their substrate is dependent primarily on the cell type and the age of the culture. Trypsins have also been used for the re-suspension of cells during cell culture, in proteomics research for digestion of proteins and in various in-gel digestions. Additional applications include assessing crystallization by membrane-based techniques and in a study to determine that protein folding rates and yields can be limited by the presence of kinetic traps.
Biochem/physiol Actions
Trypsin cleaves peptides on the C-terminal side of lysine and arginine residues. The rate of hydrolysis of this reaction is slowed if an acidic residue is on either side of the cleavage site and hydrolysis is stopped if a proline residue is on the carboxyl side of the cleavage site. The optimal pH for trypsin activity is 7-9. Trypsin can also act to cleave ester and amide linkages of synthetic derivatives of amino acids. EDTA is added to trypsin solutions as a chelating agent that neutralizes calcium and magnesium ions that obscure the peptide bonds on which trypsin acts. Removing these ions increases the enzymatic activity.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Components
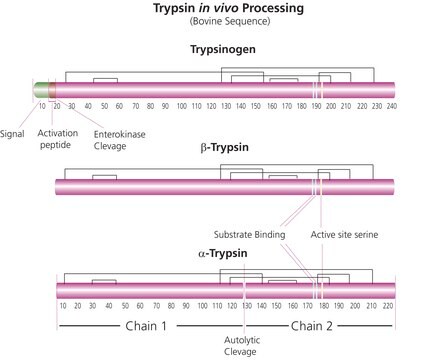
Trypsin consists of a single chain polypeptide of 223 amino acid residues, produced by the removal of the N-terminal hexapeptide from trypsinogen which is cleaved at the Lys - lle peptide bond. The sequence of amino acids is cross-linked by 6 disulfide bridges. This is the native form of trypsin, beta-trypsin. BETA-trypsin can be autolyzed, cleaving at the Lys - Ser residue, to produce alpha-trypsin. Trypsin is a member of the serine protease family.
Caution
Solutions in 1 mM HCl are stable for 1 year in aliquots and stored at -20°C. The presence of Ca2+ will also diminish the self-autolysis of trypsin and maintain its stability in solution. Trypsin will also retain most of its activity in 2.0 M urea, 2.0 M guanidine HCl, or 0.1% (w/v) SDS.
Unit Definition
One BAEE unit will produce a A253 of 0.001 per minute at pH 7.6 at 25°C using BAEE as a substrate.
Preparation Note
Soluble in 1 mM HCl at 1 mg/mL.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shuo Sui et al.
Journal of applied crystallography, 54(Pt 4), 1034-1046 (2021-08-26)
A novel capillary-based microfluidic strategy to accelerate the process of small-molecule-compound screening by room-temperature X-ray crystallography using protein crystals is reported. The ultra-thin microfluidic devices are composed of a UV-curable polymer, patterned by cleanroom photolithography, and have nine capillary channels
Monika Gulia-Nuss et al.
PloS one, 6(5), e20401-e20401 (2011-06-08)
Mosquitoes are insects that vector many serious pathogens to humans and other vertebrates. Most mosquitoes must feed on the blood of a vertebrate host to produce eggs. In turn, multiple cycles of blood feeding promote frequent contacts with hosts and
Olivier Rivoire
Physical review letters, 110(17), 178102-178102 (2013-05-18)
Studies of coevolution of amino acids within and between proteins have revealed two types of coevolving units: coevolving contacts, which are pairs of amino acids distant along the sequence but in contact in the three-dimensional structure, and sectors, which are
Mian Zhou et al.
Nature, 495(7439), 111-115 (2013-02-19)
Codon-usage bias has been observed in almost all genomes and is thought to result from selection for efficient and accurate translation of highly expressed genes. Codon usage is also implicated in the control of transcription, splicing and RNA structure. Many
Janina Boyken et al.
Neuron, 78(2), 285-297 (2013-04-30)
Neurotransmission involves calcium-triggered fusion of docked synaptic vesicles at specialized presynaptic release sites. While many of the participating proteins have been identified, the molecular composition of these sites has not been characterized comprehensively. Here, we report a procedure to biochemically
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service