P9053
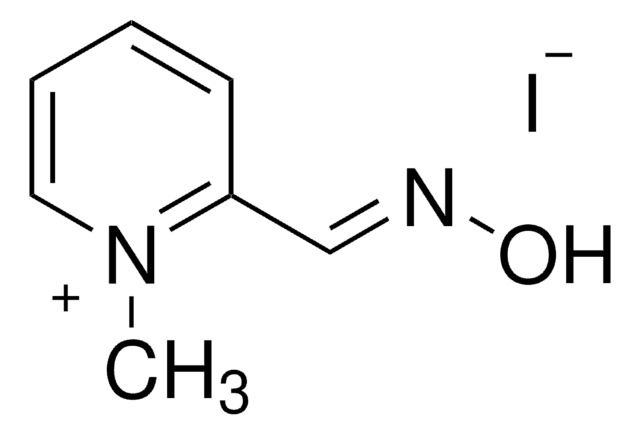
Pyridine-2-aldoxime methochloride
Synonym(s):
2-PAM chloride, Pralidoxime chloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H9N2O · Cl
CAS Number:
Molecular Weight:
172.61
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
biological source
synthetic (organic)
Quality Level
form
solid
mp
230 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
SMILES string
[Cl-].C[n+]1ccccc1\C=N\O
InChI
1S/C7H8N2O.ClH/c1-9-5-3-2-4-7(9)6-8-10;/h2-6H,1H3;1H
InChI key
HIGSLXSBYYMVKI-UHFFFAOYSA-N
Gene Information
human ... ACHE(43)
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
The prototypical reactivator of acetylcholinesterase that has been inactivated by organophosphorus insecticides or nerve agents. It is now known that no reactivator is effective against a broad spectrum of organophosphorus agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chennamaneni Srinivas Rao et al.
Bioorganic & medicinal chemistry letters, 16(8), 2134-2138 (2006-02-17)
In continuation of our investigations of unsymmetrical bisquaternary monooximes, we synthesized four new series of compounds bridged by hexyl, heptyl, octyl and nonyl groups. All eight monooximes viz., dibromides of 1-(4-hydroxyiminomethylpyridinium)6-(3/4-carbamoylpyridinium)hexane, 1-(4-hydroxyiminomethylpyridinium)-7-(3/4-carbamoylpyridinium)heptane, 1-(4-hydroxyiminomethylpyridinium)-8-(3/4-carbamoylpyridinium)octane, 1-(4-hydroxyiminomethylpyridinium)-9-(3/4-carbamoylpyridinium)nonane as well as the corresponding bis-oximes
Jyotiranjan Acharya et al.
European journal of medicinal chemistry, 46(9), 3926-3933 (2011-06-28)
A series of carbamoyl bis-pyridinium monooximes linked with xylene linker were synthesized and their in-vitro reactivation potential was evaluated against acetylcholinesterase (AChE) inhibited by organophosphorus inhibitors (OP) such as sarin, DFP and VX and the data were compared with reactivation
Kamil Musilek et al.
Bioorganic & medicinal chemistry letters, 16(21), 5673-5676 (2006-08-29)
Three asymmetrical AChE reactivators with cyano-moiety and propane linker were synthesized using modification of currently known synthetic pathways. Their potency to reactivate AChE inhibited by nerve agent tabun and insecticide paraoxon was tested in vitro and compared to pralidoxime, HI-6
Kamil Musilek et al.
Bioorganic & medicinal chemistry, 15(21), 6733-6741 (2007-09-04)
Acetylcholinesterase reactivators are crucial antidotes for the treatment of organophosphate intoxication. Fifteen new monooxime reactivators of acetylcholinesterase with a (E)-but-2-ene linker were developed in an effort to extend the properties of K-oxime (E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide (K203). The known reactivators (pralidoxime, HI-6
Kamil Musilek et al.
Journal of medicinal chemistry, 50(22), 5514-5518 (2007-10-11)
Acetylcholinesterase reactivators are crucial antidotes for the treatment of organophosphate intoxication. Among the organophosphates, with the exception of soman, tabun (GA) intoxications are the least responsive to treatment with commercially available therapeutics. A rational design was used to increase reactivation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service