D3897
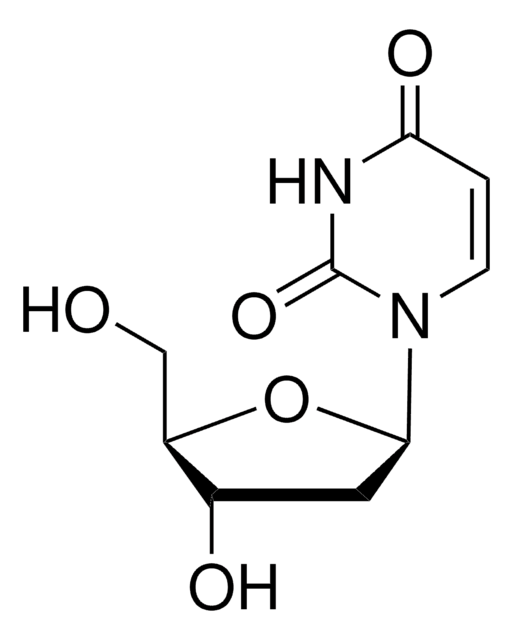
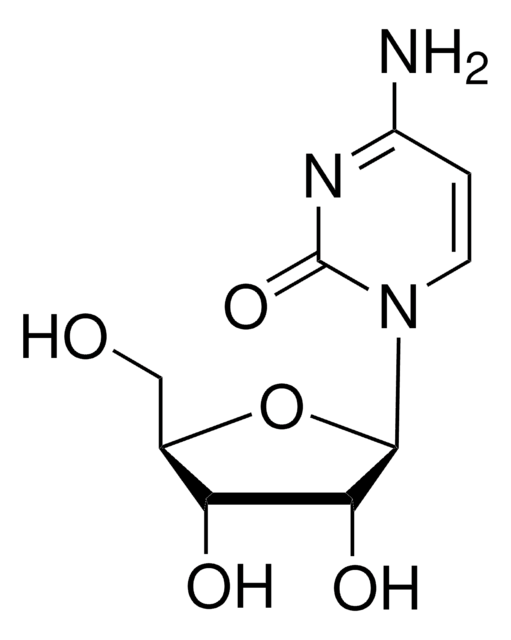
2′-Deoxycytidine
≥99% (HPLC)
Synonym(s):
Cytosine deoxyriboside
Sign Into View Organizational & Contract Pricing
All Photos(6)
About This Item
Empirical Formula (Hill Notation):
C9H13N3O4
CAS Number:
Molecular Weight:
227.22
Beilstein:
87567
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Assay
≥99% (HPLC)
form
powder
solubility
water: 50 mg/mL, clear, colorless to very faintly yellow
storage temp.
−20°C
SMILES string
NC1=NC(=O)N(C=C1)[C@H]2C[C@H](O)[C@@H](CO)O2
InChI
1S/C9H13N3O4/c10-7-1-2-12(9(15)11-7)8-3-5(14)6(4-13)16-8/h1-2,5-6,8,13-14H,3-4H2,(H2,10,11,15)/t5-,6+,8+/m0/s1
InChI key
CKTSBUTUHBMZGZ-SHYZEUOFSA-N
General description
2′-Deoxycytidine (deoxyC) is one of the deoxynucleosides containing cytosine as the nucleobase. It is found in the blood, feces, and urine.
Application
2′-Deoxycytidine has been used:
- as a substrate for Trypanosoma brucei cytidine deaminase (TbCDA) to measure its activity
- as a standard in the isolation and quantification of metabolite levels in murine tumor interstitial fluid by liquid chromatography-mass spectrometry (LC–MS)
- to study the role of autophagy in response to oncogenes and DNA replication stress
Biochem/physiol Actions
2′-Deoxycytidine (deoxyC) forms dCTP upon phosphorylation which is used to synthesis DNA via various DNA polymerases or reverse transcriptases. DeoxyC is the substrate for deoxycytidine deaminase (EC 3.5.4.14) which converts it into 2′-deoxyuridine. DeoxyC is phosphorylated to the nucleotide dCMP by the enzyme deoxycytidine kinase (DCK). DeoxyC serves as a potential head and neck cancer marker.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hiroya Murakami et al.
Talanta, 177, 12-17 (2017-11-08)
Acetaldehyde (AA), which is present in tobacco smoke, automobile exhaust gases and alcohol beverage, is a mutagen and carcinogen. AA reacts with 2'-deoxyguanosine (dG) in DNA to form N
Martyna Modrzejewska et al.
Free radical biology & medicine, 101, 378-383 (2016-11-12)
The most plausible mechanism behind active demethylation of 5-methylcytosine involves TET proteins which participate in oxidation of 5-methylcytosine to 5-hydroxymethylcytosine; the latter is further oxidized to 5-formylcytosine and 5-carboxycytosine. 5-Hydroxymethyluracil can be also generated from thymine in a TET-catalyzed process.
Annika R Seddon et al.
Epigenetics & chromatin, 14(1), 17-17 (2021-03-26)
Environmental factors, such as oxidative stress, have the potential to modify the epigenetic landscape of cells. We have previously shown that DNA methyltransferase (DNMT) activity can be inhibited by sublethal doses of hydrogen peroxide (H2O2). However, site-specific changes in DNA
Santiago Uribe-Lewis et al.
Genome biology, 16, 69-69 (2015-04-09)
The discovery of cytosine hydroxymethylation (5hmC) as a mechanism that potentially controls DNA methylation changes typical of neoplasia prompted us to investigate its behaviour in colon cancer. 5hmC is globally reduced in proliferating cells such as colon tumours and the
E Mini et al.
Annals of oncology : official journal of the European Society for Medical Oncology, 17 Suppl 5, v7-12 (2006-06-30)
Gemcitabine (2',2'-difluoro 2'-deoxycytidine, dFdC) is the most important cytidine analogue developed since cytosine arabinoside (Ara-C). The evidence of its potent antitumor activity in a wide spectrum of in vitro and in vivo tumor models has been successfully confirmed in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service