T-058
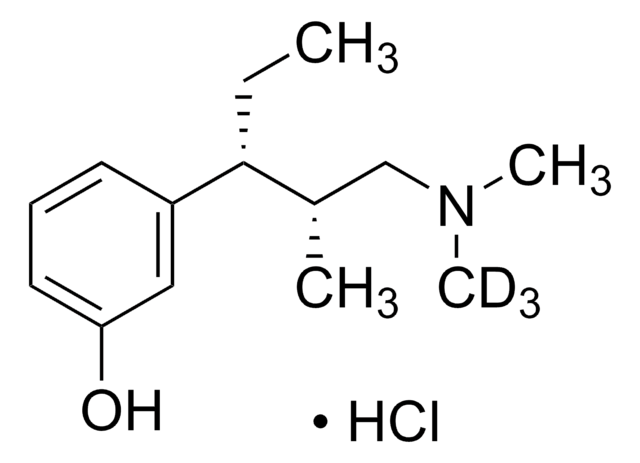
Tapentadol hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
Tapentadol hydrochloride solution
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
concentration
1.0 mg/mL in methanol (as free base)
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
−20°C
SMILES string
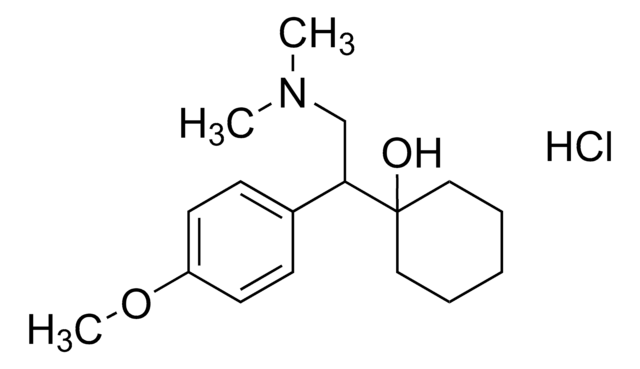
Cl.CC[C@H]([C@@H](C)CN(C)C)c1cccc(O)c1
InChI
1S/C14H23NO.ClH/c1-5-14(11(2)10-15(3)4)12-7-6-8-13(16)9-12;/h6-9,11,14,16H,5,10H2,1-4H3;1H/t11-,14+;/m0./s1
InChI key
ZELFLGGRLLOERW-YECZQDJWSA-N
Gene Information
human ... OPRM1(4988) , SLC6A2(6530)
General description
Application
- Extended-Release Formulation for Chronic Pain: Extended-release formulations of Tapentadol hydrochloride are developed to provide sustained pain relief for chronic conditions. This formulation reduces the frequency of dosing and enhances patient compliance, offering consistent pain management (Faria et al., 2017).
- Pharmaceutical Quality Control and Research: High-purity Tapentadol hydrochloride solutions are essential in pharmaceutical research and quality control. These solutions are used for the calibration of analytical instruments and ensuring the consistency and safety of pharmaceutical products (Fejos et al., 2014).
- Transdermal Delivery Systems: Innovative research has developed PEGylated ultra-deformable transferosomes for the transdermal delivery of Tapentadol, improving its bioavailability and analgesic activity. This method offers a non-invasive alternative for pain management (Deng et al., 2022).
- Innovative Pain Management Solutions: Research on new delivery methods, such as intranasal administration using chitosan nanoparticles, aims to enhance the therapeutic potential and patient compliance of Tapentadol hydrochloride solution for pain management (Javia & Thakkar, 2017).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
-THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service