N1501
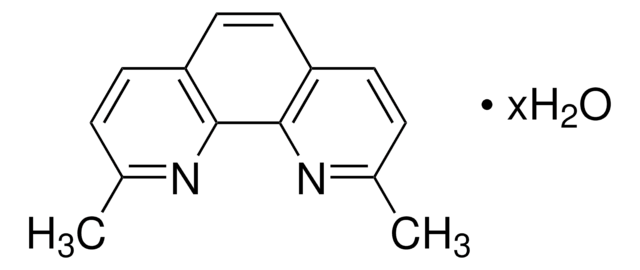
Neocuproine
≥98%
Synonym(s):
2,9-Dimethyl-1,10-phenanthroline, DMPHEN
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12N2
CAS Number:
Molecular Weight:
208.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
crystalline
color
white to beige
SMILES string
Cc1ccc2ccc3ccc(C)nc3c2n1
InChI
1S/C14H12N2/c1-9-3-5-11-7-8-12-6-4-10(2)16-14(12)13(11)15-9/h3-8H,1-2H3
InChI key
IYRGXJIJGHOCFS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Neocuproine can be used as a ligand:
- In the aerobic oxidation of benzyl and allylic alcohols to corresponding carbonyl compounds using Au(I) as a catalyst.
- To synthesize aqua(2,9-dimethyl-1,10-phenanthroline)NiCl2 complex, which is used as a precursor for the preparation of uniform spherical NiO nanoparticles via the thermal decomposition method.
- In the Zn-catalyzed allylation reactions of aldehydes with allyl boronates to prepare α-addition products with high diastereoselectivities.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mustafa Ozyürek et al.
Analytica chimica acta, 616(2), 196-206 (2008-05-17)
Hydroxyl radicals (OH) generated in the human body may play an important role in tissue injury at sites of inflammation in oxidative stress-originated diseases. As a more convenient, efficient, and less costly alternative to HPLC/electrochemical detection techniques and to the
Saliha Esin Celik et al.
Analytica chimica acta, 674(1), 79-88 (2010-07-20)
A novel on-line HPLC-cupric reducing antioxidant capacity (CUPRAC) method was developed for the selective determination of polyphenols (flavonoids, simple phenolic and hydroxycinnamic acids) in complex plant matrices. The method combines chromatographic separation, constituent analysis, and post-column identification of antioxidants in
Yan Wang et al.
Journal of the American Society for Mass Spectrometry, 19(9), 1353-1360 (2008-07-19)
S-nitrosylation of proteins serves an important role in regulating diverse cellular processes including signal transduction, DNA repair, and neurotransmission. Identification of S-nitrosylation sites is crucial for understanding the significance of this post-translational modification (PTM) in modulating the function of a
Mustafa Bener et al.
Analytical chemistry, 82(10), 4252-4258 (2010-04-27)
A low-cost optical sensor using an immobilized chromogenic redox reagent was devised for measuring the total antioxidant level in a liquid sample without requiring sample pretreatment. The reagent, copper(II)-neocuproine (Cu(II)-Nc) complex, was immobilized onto a cation-exchanger film of Nafion, and
Sung-Ho Chen et al.
Toxicological sciences : an official journal of the Society of Toxicology, 102(1), 138-149 (2007-12-07)
Astrocytes play a critical neurotrophic and neuroprotective role in the brain, and improper function of these cells may contribute to the onset of neurodegenerative diseases. Because astrocytes are known to be enriched with Cu chaperone proteins, it is important to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service