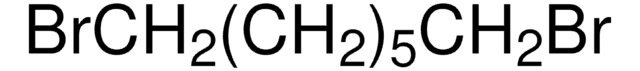D41007
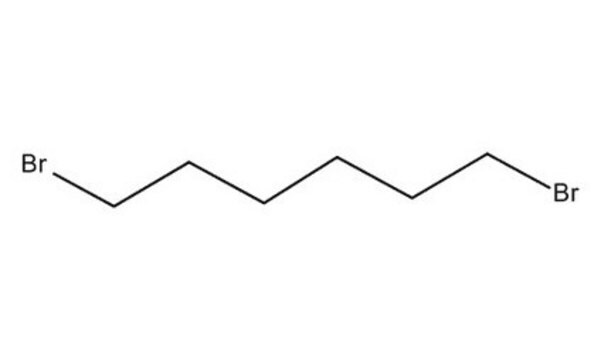
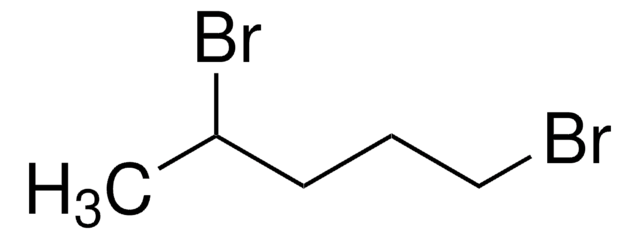
1,6-Dibromohexane
96%
Synonym(s):
Hexamethylene dibromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)6Br
CAS Number:
Molecular Weight:
243.97
Beilstein:
1236322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.507 (lit.)
bp
243 °C (lit.)
mp
−2-2.5 °C (lit.)
density
1.586 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCBr
InChI
1S/C6H12Br2/c7-5-3-1-2-4-6-8/h1-6H2
InChI key
SGRHVVLXEBNBDV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,6-Dibromohexane is classified as an alkyl halide compound and is useful as a versatile building block in the synthesis of diverse organic compounds.
Application
1,6-Dibromohexane is generally used to introduce C6 spacer in the molecular architecture. Some of the examples are:
- Synthesis of solvent processable and conductive polyfluorene ionomers for alkaline fuel cell applications.
- Synthesis of cross-linkable regioregular poly(3-(5-hexenyl)thiophene) (P3HNT) for stabilizing the film morphology in polymer photovoltaic cells.
- Synthesis of pyrrolo-tetrathiafulvalene molecular bridge (6PTTF6) to study redox switching behavior of single molecules.
- Synthesis of water-soluble thermoresponsive polylactides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structure? Property Relationships in Redox-Gated Single Molecule Junctions? A Comparison of Pyrrolo-Tetrathiafulvalene and Viologen Redox Groups.
Leary E, et al.
Journal of the American Chemical Society, 130(37), 12204-12205 (2008)
Adam S Mullis et al.
Molecular pharmaceutics, 16(5), 1917-1928 (2019-04-12)
Drug delivery vehicles can improve the functional efficacy of existing antimicrobial therapies by improving biodistribution and targeting. A critical property of such nanomedicine formulations is their ability to control the release kinetics of their payloads. The combination of (and interactions
Water-soluble thermoresponsive polylactides.
Jiang X, et al.
Macromolecules, 41(2), 318-324 (2008)
A soluble and conductive polyfluorene ionomer with pendant imidazolium groups for alkaline fuel cell applications.
Lin B, et al.
Macromolecules, 44(24), 9642-9649 (2011)
Yashdeep Phanse et al.
Journal of biomedical materials research. Part A, 105(10), 2762-2771 (2017-05-31)
Rational design of adjuvants and delivery systems will promote development of next-generation vaccines to control emerging and re-emerging diseases. To accomplish this, understanding the immune-enhancing properties of new adjuvants relative to those induced by natural infections can help with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service