All Photos(3)
About This Item
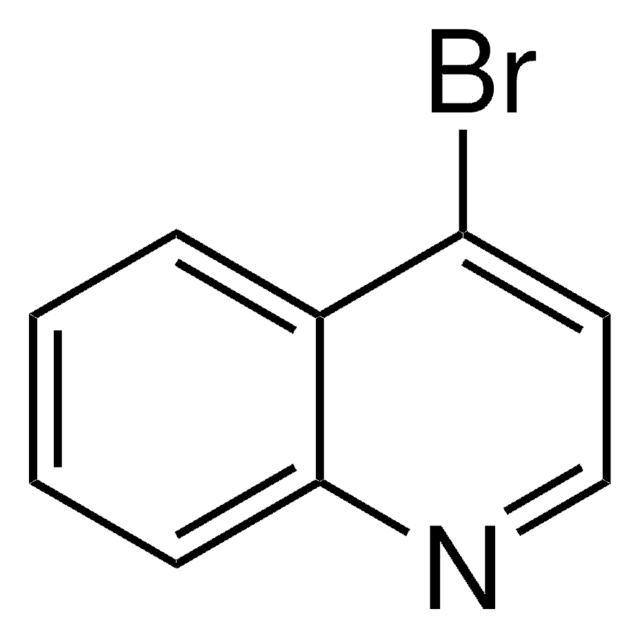
Empirical Formula (Hill Notation):
C9H6ClN
CAS Number:
Molecular Weight:
163.60
Beilstein:
112561
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
bp
266-267 °C (lit.)
mp
34-37 °C (lit.)
density
1.23 g/mL at 25 °C (lit.)
SMILES string
Clc1ccc2ccccc2n1
InChI
1S/C9H6ClN/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
InChI key
OFUFXTHGZWIDDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Shiva Kumar et al.
Bioorganic & medicinal chemistry, 20(7), 2199-2207 (2012-03-06)
A number of 2-(1H-indol-3-yl)quinoline-3-carbonitrile derivatives were synthesized via AlCl(3)-mediated C-C bond forming reaction between 2-chloroquinoline-3-carbonitrile and various indoles. The methodology does not require any N-protection of the indoles employed and provided the corresponding products in good yields. The molecular structure
Santosh Kumar et al.
International journal of biological macromolecules, 49(3), 356-361 (2011-06-07)
This paper describes an elegant cross-linking technique for the preparation of chitosan-chloroquinoline derivative by using a greener technique. Chitosan solution in aqueous acetic acid was treated with 2-chloroquinoline-3-carbaldehyde solution to form hydrogel; the resulting hydrogel was subjected to solvent exchange.
S Fetzner et al.
FEMS microbiology letters, 112(2), 151-157 (1993-09-01)
Resting cells of Pseudomonas putida strain 86 were grown on quinoline transformed 2-chloroquinoline to 2-chloro-cis-7,8-dihydro-7,8-dihydroxyquinoline which was not converted further. 7,8-Dioxygenating activity was present when the enzymes of quinoline catabolism were induced. Quinoline-grown cells of strain 86 treated simultaneously with
Krzysztof Marciniec et al.
Journal of chromatographic science, 55(9), 934-939 (2017-06-28)
The lipophilicity of a series of anticancer propargylquinoline derivatives is investigated using both chromatographic and computational methods. The parameters of the tested compounds' relative lipophilicity (logkw) are determined experimentally by the high-performance liquid chromatographic method (RP-HPLC, Accucore C18 column), using
D R Boyd et al.
Organic & biomolecular chemistry, 8(5), 1081-1090 (2010-02-19)
A series of enantiopure 2,2'-bipyridines have been synthesised from the corresponding cis-dihydrodiol metabolites of 2-chloroquinolines. Several of the resulting hydroxylated 2,2'-bipyridines were found to be useful chiral ligands for the asymmetric aminolysis of meso-epoxides leading to the formation of enantioenriched
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service