A86805
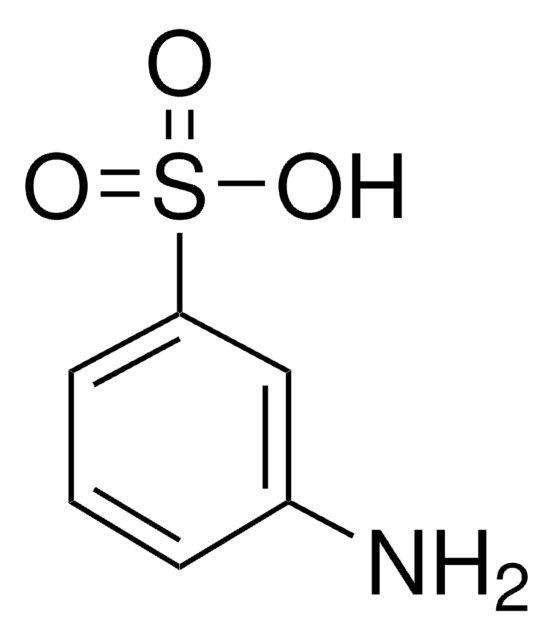
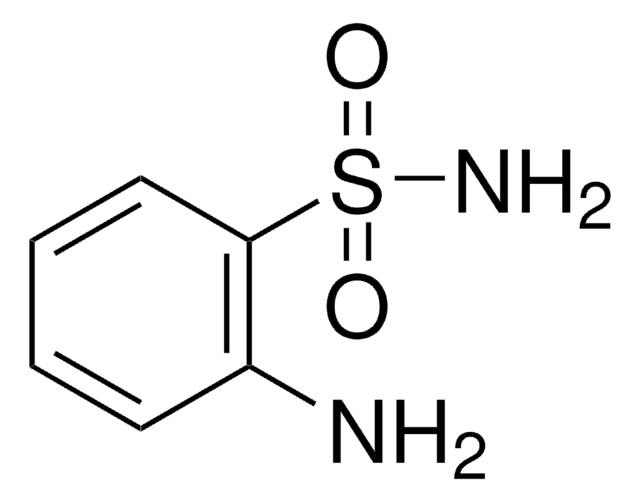
Aniline-2-sulfonic acid
95%
Synonym(s):
Orthanilic acid, 2-Aminobenzenesulfonic acid, o-Sulfanilic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4SO3H
CAS Number:
Molecular Weight:
173.19
Beilstein:
1309204
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
SMILES string
Nc1ccccc1S(O)(=O)=O
InChI
1S/C6H7NO3S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H,8,9,10)
InChI key
ZMCHBSMFKQYNKA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sangram S Kale et al.
Chemical communications (Cambridge, England), 49(22), 2222-2224 (2013-02-09)
Orthanilic acid (2-aminobenzenesulfonic acid, (S)Ant), an aromatic β-amino acid, has been shown to be highly useful in inducing a folded conformation in peptides. When incorporated into peptide sequences (Xaa-(S)Ant-Yaa), this rigid aromatic β-amino acid strongly imparts a reverse-turn conformation to
F Junker et al.
Microbiology (Reading, England), 140 ( Pt 7), 1713-1722 (1994-07-01)
Alcaligenes sp. strain O-1 utilizes three sulphonated aromatic compounds as sole sources of carbon and energy for growth in minimal salts medium-benzenesulphonate (BS), 4-toluenesulphonate (TS) and 2-aminobenzenesulphonate (2AS). The degradative pathway(s) in 2AS-grown cells are initiated with membrane transport, NADH-dependent
Jürgen Ruff et al.
Microbiological research, 165(4), 288-299 (2009-07-07)
Alcaligenes sp. strain O-1 inducibly deaminates 2-aminobenzenesulfonate (ABS) via dioxygenation to 3-sulfocatechol, which is desulfonated during meta ring-cleavage to yield 2-hydroxymuconate. This intermediate is transformed through the oxalocrotonate-branch of the sulfocatechol meta-pathway (Scm). The complete pathway is encoded on the
Determination of nitrite ion and sulfanilic and orthanilic acids by differential pulse polarography.
S T Sulaiman
Analytical chemistry, 56(13), 2405-2407 (1984-11-01)
Effect of taurine, L-cysteic and orthanilic acids on cardiac tension.
F Franconi et al.
Progress in clinical and biological research, 351, 175-184 (1990-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service