914088
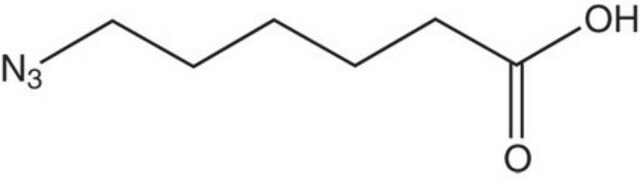
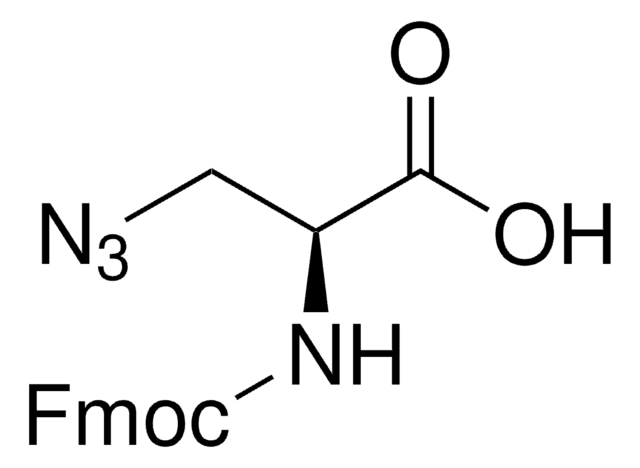
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride
≥95%
Synonym(s):
(S)-2-amino-6-((2-azidoethoxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(EO-N3)-OH HCl, Lysine-azide, UAA crosslinker
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H17N5O4 · xHCl
CAS Number:
Molecular Weight:
259.26 (free base basis)
UNSPSC Code:
12352209
Recommended Products
Application
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride is a clickable amino acid derivative for site-specific incorporation into recombinant proteins or synthesis of chemical probes and tools for biological applications. This non-canonical lysine possesses an azide for bioorthogonal reaction with alkynes.
Other Notes
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Siqi Zheng et al.
Angewandte Chemie (International ed. in English), 53(25), 6449-6453 (2014-05-16)
Coupling the genetic code expansion technique with bioorthogonal reactions enables precise control over the conjugation site as well as the choice of fluorescent probes during protein labeling. However, the advantages of this strategy over bulky and rigid fluorescent proteins (FPs)
Chuanling Zhang et al.
Biomaterials, 80, 134-145 (2015-12-29)
Virus-based nanoparticles have shown promise as vehicles for delivering therapeutic genes. However, the rational design of viral vectors that enable selective tropism towards particular types of cells and tissues remains challenging. Here, we explored structural-functional relationships of the adeno-associated virus
Sanggil Kim et al.
Bioorganic & medicinal chemistry, 24(22), 5816-5822 (2016-10-26)
Proteins often function as complex structures in conjunction with other proteins. Because these complex structures are essential for sophisticated functions, developing protein-protein conjugates has gained research interest. In this study, site-specific protein-protein conjugation was performed by genetically incorporating an azide-containing
Michael P VanBrunt et al.
Bioconjugate chemistry, 26(11), 2249-2260 (2015-09-04)
Antibody-drug conjugates (ADC) have emerged as potent antitumor drugs that provide increased efficacy, specificity, and tolerability over chemotherapy for the treatment of cancer. ADCs generated by targeting cysteines and lysines on the antibody have shown efficacy, but these products are
Yiming Wu et al.
Bioconjugate chemistry, 27(10), 2460-2468 (2016-10-21)
Radioimmunotherapy (RIT) delivers radioisotopes to antigen-expressing cells via monoantibodies for the imaging of lesions or medical therapy. The chelates are typically conjugated to the antibody through cysteine or lysine residues, resulting in heterogeneous chelate-to-antibody ratios and various conjugation sites. To
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service