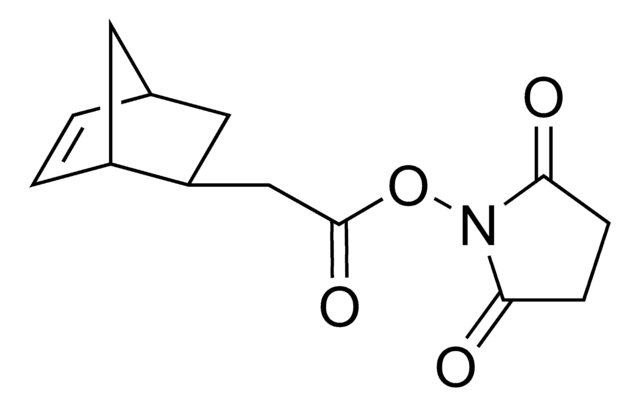
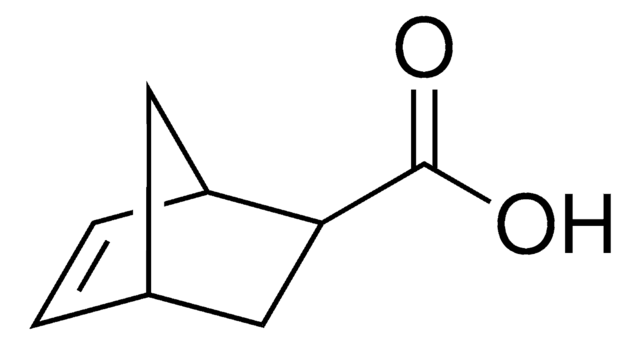
718149
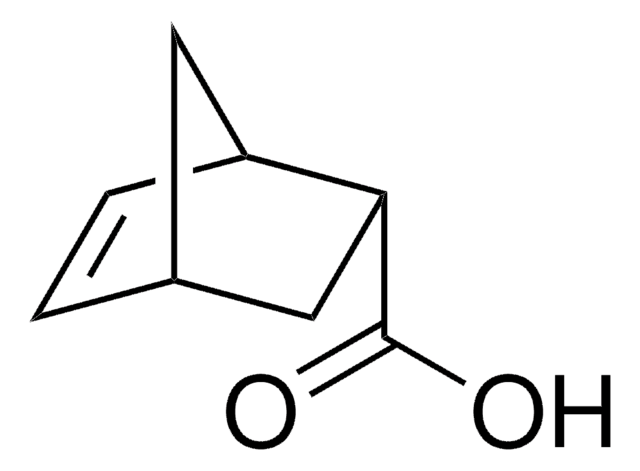
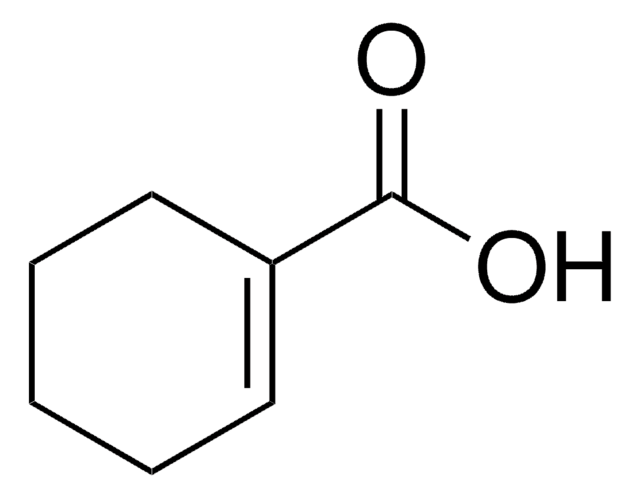
exo-5-Norbornenecarboxylic acid
97%
Synonym(s):
(1R,2S,4R)-Bicyclo[2.2.1]hept-5-ene-2-carboxylic acid, NC
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H10O2
CAS Number:
Molecular Weight:
138.16
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
mp
40-44 °C
SMILES string
OC(=O)[C@@H]1C[C@@H]2C[C@H]1C=C2
InChI
1S/C8H10O2/c9-8(10)7-4-5-1-2-6(7)3-5/h1-2,5-7H,3-4H2,(H,9,10)/t5-,6+,7+/m0/s1
InChI key
FYGUSUBEMUKACF-RRKCRQDMSA-N
General description
Exo-5-norbornenecarboxylic acid is a bicyclic compound that has potential applications in the field of material science due to its unique chemical properties. It is a versatile building block for the synthesis of various functional materials, including polymers, dendrimers, and self-assembled monolayers. It can be used to modify surfaces or to functionalize nanoparticles, influencing their optical, magnetic, or electronic properties. It is also often used in ring-opening metathesis polymerization reactions to form polymers with controlled molecular weight and structure. Additionally, exo-5-norbornenecarboxylic acid can function as a ligand for coordination chemistry and catalysis.
Application
Exo-5-norbornenecarboxylic acid can be used as:
- A starting material in the synthesis of the metathesis polymer via a ring-opening metathesis polymerization (ROMP) reaction of the ester of exo-5-norbornenecarboxylic acid and 1,1′-bi-2-naphthol.
- A monomer in the preparation of thin films via surface-initiated polymerization process. The resulting thin film serves as a template for selective deposition and etching of metal oxides, which is of significant importance in the microelectronic industry.
- Different crosslinkers for ring-opening metathesis polymerization.
- Norbornene-functionalized monomers, which are used to make poly(norbornene)s via ring-opening metathesis polymerization (ROMP).
- Hydrolytically cleavable and hydrolytically stable functionalized macromonomers for hydrogel preparation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christopher L McGann et al.
Macromolecular bioscience, 16(1), 129-138 (2015-10-06)
A range of chemical strategies have been used for crosslinking recombinant polypeptide hydrogels, although only a few have employed photocrosslinking approaches. Here, we capitalize on the novel insect protein, resilin, and the versatility of click reactions to introduce a resilin-like
Nilwala Kottegoda et al.
ACS nano, 11(2), 1214-1221 (2017-01-26)
While slow release of chemicals has been widely applied for drug delivery, little work has been done on using this general nanotechnology-based principle for delivering nutrients to crops. In developing countries, the cost of fertilizers can be significant and is
Jürgen Herrler et al.
Magnetic resonance in medicine, 85(6), 3140-3153 (2021-01-06)
To mitigate spatial flip angle (FA) variations under strict specific absorption rate (SAR) constraints for ultra-high field MRI using a combination of universal parallel transmit (pTx) pulses and fast subject-specific optimization. Data sets consisting of B0 , B 1 +
Wenjun Zheng et al.
BMC structural biology, 9, 45-45 (2009-07-14)
It is increasingly recognized that protein functions often require intricate conformational dynamics, which involves a network of key amino acid residues that couple spatially separated functional sites. Tremendous efforts have been made to identify these key residues by experimental and
Manuel Gregoritza et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 127, 194-203 (2018-02-23)
Reducing burst effects, providing controlled release, and safeguarding biologics against degradation are a few of several highly attractive applications for microgels in the field of controlled release. However, the incorporation of proteins into microgels without impairing stability is highly challenging.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service